A kind of single-chain antibody targeting EGFR dimerization interface and its application
A single-chain antibody, interface technology, applied in the direction of antibodies, applications, antibody mimics/scaffolds, etc., can solve the problems of large molecular weight, influence of tumor inhibition effect, poor penetration, etc., and achieves low molecular weight, friendly production environment, The effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 Construction of genes for single-chain antibodies and optimization of codons
[0127] 1. Acquisition of EGFR dimerization interface targeting monoclonal antibody light chain gene and heavy chain gene
[0128] 1. Experimental operation
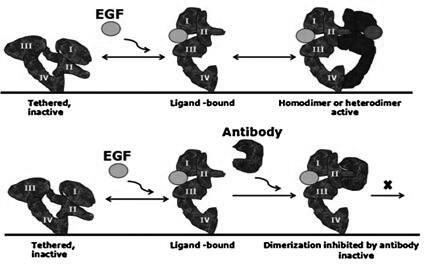
[0129] The inventor's previous research has obtained the monoclonal antibody Antidimer 5G9 that can target the dimerization interface of EGFR, and preserved its hybridoma cells.
[0130] Hybridoma cells targeting the EGFR dimerization interface were cultured, cells were collected, and RNA was extracted by Trizol method. After reverse transcription, use universal degenerate primers to amplify the heavy chain variable region and light chain variable region genes of the EGFR dimerization interface monoclonal antibody Antidimer 5G9 respectively, and clone them into the cloning vector pMD18-T vector by AT cloning superior. Sent to the sequencing company for sequencing analysis. The sequence was further optimized for Pichia pasto...
Embodiment 2
[0141] Example 2 Construction of Fusion Gene Recombinant Expression Vector of Single-chain Antibody EGFR-scFv
[0142] 1. Experimental operation
[0143] 1. PCR amplification of single chain antibody
[0144] Escherichia coli containing the fusion gene of the single-chain antibody EGFR-scFv (EGFR-scFv gene) was inoculated into 2ml of LB medium (containing 0.1% AMP), and cultured at 37°C and 180rpm for 14-16h to amplify the bacteria. Using the amplified Escherichia coli containing the single-chain antibody gene to extract the plasmid DNA therein, use it as a template strand for PCR amplification for PCR amplification.
[0145] The upstream primer for PCR amplification is: 5'-GACA CTCGAG AAGAGAGAGGCTGAGGC-3', the downstream primer is: 5'-ACTC TCTAGA TCAGTGGTGGTGGTGG-3'. Increased 5' upstream of the EGFR-scFv gene wxya Restriction site, 3' downstream increase wxya Restriction sites, and add protective bases at both ends.
[0146] PCR amplification system preparation: ...
Embodiment 3
[0171] Extraction and yeast transformation of embodiment 3 recombinant plasmids
[0172] 1. Experimental operation
[0173] 1. Extraction of recombinant plasmids
[0174] Shake 100ml of the recombinant, extract the pGAPZαA-EGFR-scFv plasmid (about 20 μg) with a small extraction kit, and linearize the recombinant plasmid with BlnI.
[0176]
[0177] Enzyme digested in a water bath at 37°C for 2 hours, and samples were taken for gel electrophoresis detection. The resulting enzyme digestion supplies were desalted with phenol-chloroform. method:
[0178] (1) with ddH 2 O Make up 500 μl of the digested plasmid, add 500 μl of phenol-chloroform-isoamyl alcohol and mix by inverting up and down. Centrifuge at 4°C and 10,000rpm for 5min, carefully absorb the supernatant, and transfer to a 2ml EP tube.
[0179] (2) Add 3M NaAc to the sample at a ratio of 10:1 between the sample and NaAc (pH5.2), then add 2 times the volume of -20°C pre-cooled abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com