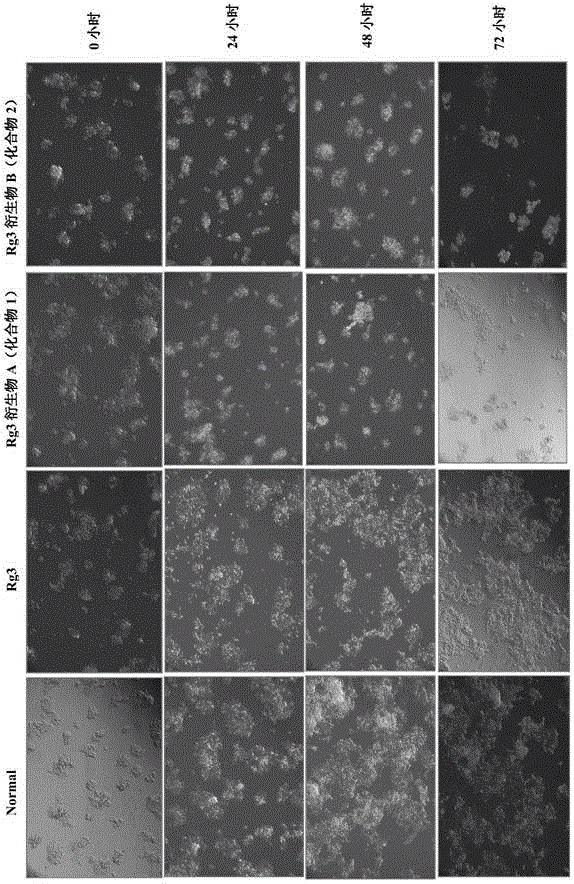
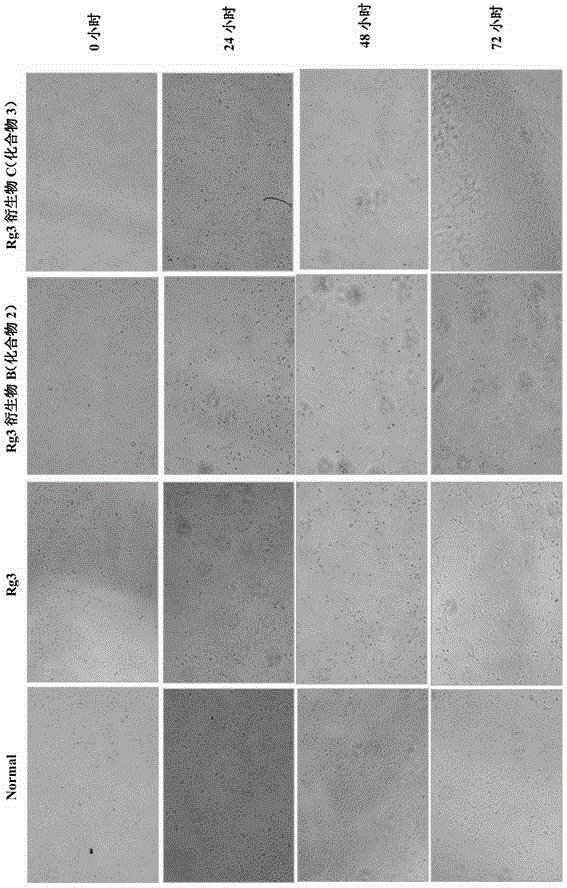
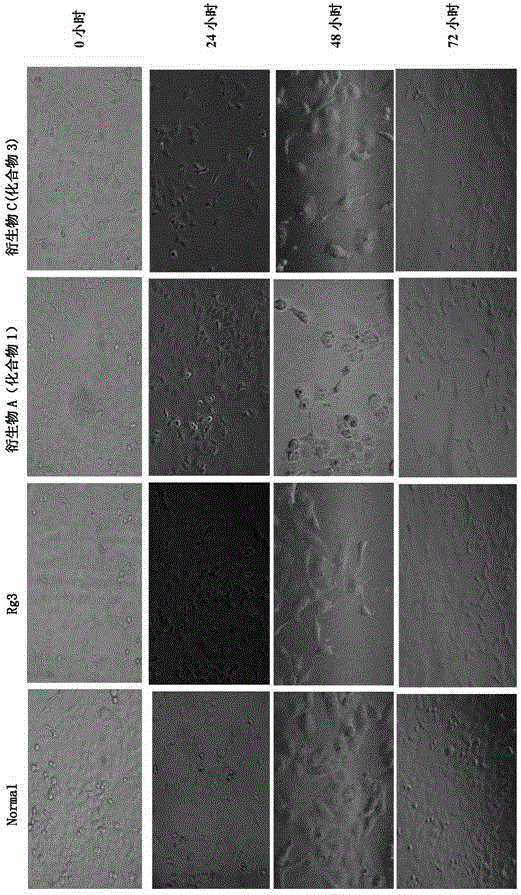
20(R)-ginsenoside Rg3 derivatives, and preparation method and application thereof
A technology of ginsenosides and derivatives, applied in chemical instruments and methods, drug combinations, glycoside steroids, etc., can solve the problems of unproven tumor inhibitory effect, no synergistic effect, and equivalent inhibitory effect, and achieve the extension zone The effect of improving tumor survival time, improving life extension rate, and increasing tumor suppression rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1. Add 20(R)-ginsenoside Rg3 (4g, 5.09mmol) into 180ml of dry anhydrous pyridine, stir and dissolve to prepare 20(R)-ginsenoside Rg3 solution;
[0066] In addition to anhydrous pyridine, the organic solvent for dissolving 20(R)-ginsenoside Rg3 in the embodiment of the present invention can also be anhydrous 2,4,6-collidine or anhydrous 2,6-dimethyl pyridine.
[0067] 2. Under the condition of ice bath (0°C), feed nitrogen into the reaction vessel, remove the air, prevent the moisture in the air from contacting the acid chloride reagent, and cause the denaturation of the acid chloride, to 20(R)-ginsenoside Rg3 Add palmitoyl chloride (3.07ml, about 10mmol) dropwise in the solution, stir while adding dropwise, the rate of addition is 1 drop (about 50ul / drop) / 2s (promptly add to 20(R)-ginseng at a rate of one drop per 2 seconds Saponin Rg3 solution is added dropwise with palmitoyl chloride), until the dropwise addition of palmitoyl chloride is completed to obtain a ginseno...
Embodiment 2
[0095] 1. Add 20(R)-ginsenoside Rg3 (4g, 5.09mmol) into 180ml of dry anhydrous pyridine, stir and dissolve to prepare 20(R)-ginsenoside Rg3 solution;
[0096] 2. Under the condition of ice bath (-10°C), feed nitrogen into the reaction vessel to remove the air, prevent the moisture in the air from contacting with the acid chloride reagent, and under the condition of denaturation of the acid chloride, add 20(R)-ginsenoside Rg3 Add palmitoyl chloride (3.07ml, about 10mmol) dropwise in the solution, stir while adding dropwise, the rate of addition is 1 drop / 2s, until the palmitoyl chloride is added dropwise, the ginsenoside-acyl chloride mixture is obtained; wherein, 20(R)- The molar ratio of ginsenoside Rg3 to palmitoyl chloride is 1:1.9.
[0097] In the embodiment of the present invention, the dropping rate in the process of dropping the acylating reagent acid chloride is described by taking 1 drop / 2s as an example, and other dropping rates such as 1 drop / 1-5s are applicable to ...
Embodiment 3
[0103] 1. Add 20(R)-ginsenoside Rg3 (4g, 5.09mmol) into 180ml of dry anhydrous pyridine, stir and dissolve to prepare 20(R)-ginsenoside Rg3 solution;
[0104] 2. Under the condition of ice bath (0°C), feed nitrogen into the reaction vessel, remove the air, prevent the moisture in the air from contacting the acid chloride reagent, and cause the denaturation of the acid chloride, to 20(R)-ginsenoside Rg3 Add palmitoyl chloride (3.07ml, about 10mmol) dropwise in the solution, stir while adding dropwise, the rate of addition is 1 drop / 2s, until the palmitoyl chloride is added dropwise, the ginsenoside-acyl chloride mixture is obtained; wherein, 20(R)- The molar ratio of ginsenoside Rg3 to stearyl chloride is 1:2.3;
[0105] 3. Under the condition of feeding inert gas (nitrogen) into the reaction vessel all the time, after adding the acid chloride reagent dropwise, heat it under stirring to raise the temperature of the ginsenoside-acyl chloride mixture to 5°C, and keep the temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com