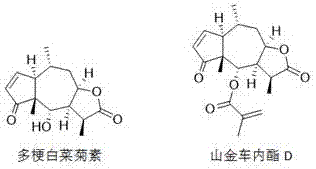
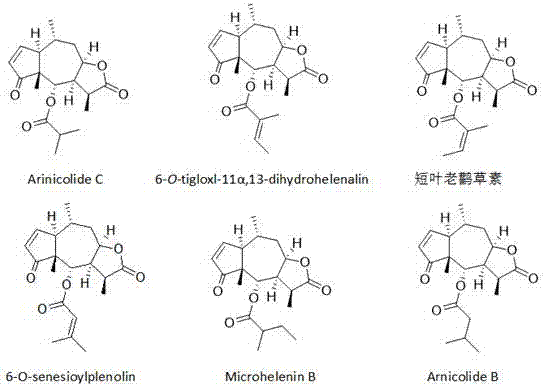
Application of sesquiterpene lactone compounds in the preparation of anti-influenza virus drugs
An ester compound, anti-influenza virus technology, applied in the field of medicine, achieves the effects of easy availability of raw materials, good anti-influenza virus activity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Grind 300g of dried goose-free grass, pass through a 80-mesh sieve, and place in supercritical CO 2 In the extractor, the extraction pressure is 20 MPa, the extraction temperature is 30°C, the flow rate is 15 liters / hour, and the extraction time is 2 hours, and 14.8 g of supercritical carbon dioxide extract of goose and herbivorous are obtained;
[0020] Dissolve the supercritical carbon dioxide extract of Goose Bubiograss in ethyl acetate, mix the sample with 12g of silica gel (100-200 mesh), and evaporate to dryness under reduced pressure to obtain the sample; take another 90g of silica gel (200-300 mesh) for dry packing Column (inner diameter × length = 3.5 × 50cm), put the sample on the column, then cover with a layer of silica gel, first elute with petroleum ether, and then use petroleum ether and ethyl acetate (volume ratio: 7.5:1.5) Mixed solvent elution, collecting the mixed solvent eluted part, concentrating and drying to obtain 2.4 g of the mixed solvent elute...
Embodiment 2
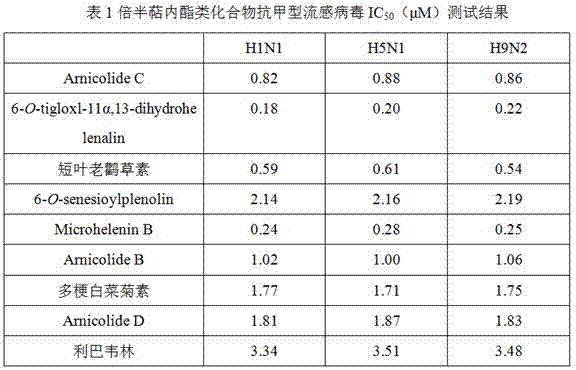
[0023] Example 2 Anti-influenza A virus activity test of sesquiterpene lactones
[0024] Take a 96-well cell culture plate covered with a monolayer of MDCK cells, discard the supernatant, wash twice with PBS, add 100TCID 50 The virus solution was 100 μL / well, and was adsorbed for 1 h, and then 100 μL of the drug solution diluted with MEM was added to each well. In addition, a cell control group, a virus control group, and a blank control group were set up, and ribavirin was used as a positive control drug. Placed at 37°C, saturated humidity, 5% CO 2 Incubate in the incubator. After 48 h, the old solution in the 96-well plate was discarded, and the original solution of CCK8 was diluted ten times and added to the tested wells at 100 µL per well. After incubation in the incubator for 1 h, the absorbance was measured at 450 nm with a microplate reader. IC 50 The calculation method is as follows: cell survival rate = (OD value of the drug-dosing well - OD value of the virus con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com