Application of usnic acid in anti-influenza virus medicaments
An anti-influenza virus and influenza virus technology, applied in the field of medicine, can solve problems such as the absence of usnic acid, and achieve the effects of alleviating treatment pain, improving treatment quality, medication compliance, and improving tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
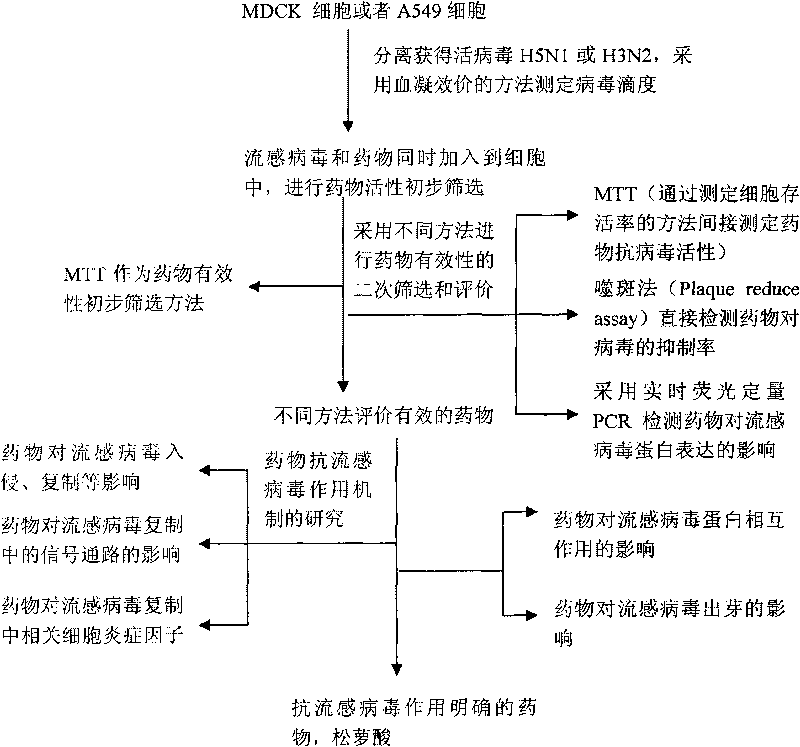
[0042] Embodiment 1: Evaluation of usnic acid anti-influenza virus activity
[0043] I. Test material
[0044] 1. Cells, viruses and plasmids:
[0045] MDCK cells were provided by Shandong Institute of Medical Microbiology, Chinese Academy of Sciences; A549 cells, HeLa cells, virus H5N1 or H3N2 were purchased from China Collection Center for Typical Microorganisms; pM-scFv, pVP-16-NP, pM-PB1, pM-PB2, pVP- The 16-NP and pG5Luc plasmids were completed under the guidance of Professor Wu Jianguo from the Medical Virology Research Group of the State Key Laboratory of Virology, Wuhan University. For detailed information, please refer to the published papers of this laboratory—Mukhtar, M.M., Li, S., Li, W., Wan, T., Mu, Y., Wei, W., Kang, L., Rasool, S.T., Xiao, Y., Zhu, Y., and Wu, J. (2009). Single-chain intracellular Antibodies inhibit influenza virus replication by disrupting interaction of proteins involved in viral replication and transcription. Int J Biochem Cell Biol 41(3),...
Embodiment 2
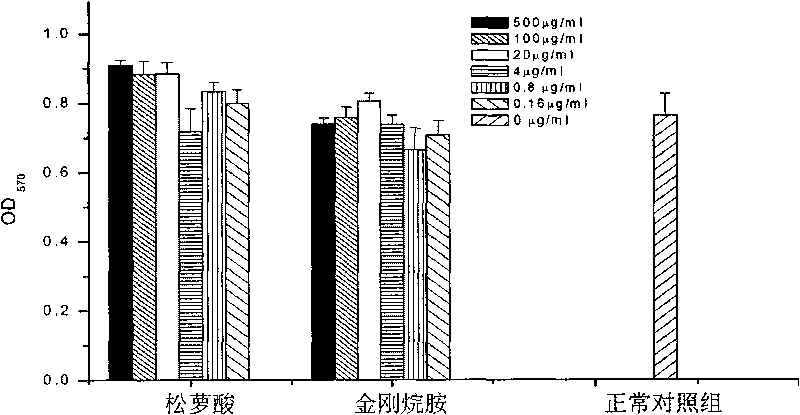
[0147] Example 2: Usnic acid prevents viral infection, and influences on influenza virus adsorption invasion and replication
[0148] The cells paved into a single layer were treated in different ways: the drug was added to the cells in advance and incubated for 1 hour, the medium was changed, and the corresponding influenza virus was added; the drug was added to the virus for adsorption and invasion of the influenza virus, and the drug was added to the virus and incubated for 1 hour, and then Then add it to the cells; for the effect of the drug on the replication of the influenza virus, the virus is first used to infect the cells for 1 hour, and then the drug is added. The drug concentration used in all experiments was 100 μg / ml, and the virus infection amount was 1M.O.I virus liquid. Part of the supernatant was collected at 24h, 36h, and 48h respectively, and the antiviral effect of the drug was determined by HA.
[0149] see results Figure 9-1 , 9-2 and 9-3, indicating ...
Embodiment 3
[0150] Embodiment 3: The influence of usnic acid on some enzymes in the replication process is explored by two-hybrid experiments in mammals
[0151] (1) Cell transfection
[0152] The plasmids are pM-PB1, pM-PB2, pVP-16-NP, pG5Luc, respectively. In this test group, no plasmids were added to the negative control, pM-PB1 (or pM-PB2), pVP-16-NP, and pG5Luc were added to the positive control, and pM-PB1 (or pM-PB2), pVP- 16-NP, pG5Luc three plasmids and various drugs.
[0153] Sofast TM lipofection
[0154] The following transfection operation uses a 24-well plate as an example. The whole process of transfection must be strictly aseptic.
[0155] 1. Seed the cells to be transfected in a 24-well plate one day before transfection and culture at 37°C. The cell density before transfection was 40-60%.
[0156] 2. Prepare the following solutions
[0157] ① Dilute 1.5μl Sofast TM Reagents in 30 μl serum-free, antibiotic-free DMEM medium. After mixing, let stand at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com