The use of notoginseng saponin ft1
A technology of Panax notoginseng saponins and uses, applied in the field of medicine, can solve problems such as recurrent attacks, achieve the effects of improving weight loss and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Experimental materials
[0043] 1.1 Medicinal materials
[0044] Notoginseng saponin Ft1 (HPLC purity ≥98%, Chengdu Master Biotechnology Co., Ltd.); Dextran sodium sulfate (DSS, CAS: 9011-18-1, MW36-50kDa, produced by MP Biomedicals, USA); Willow Azasulfapyridine (SASP, CAS: 599-79-1, molecular weight 398.39, HPLC purity ≥ 98%, produced by Sigma-Aldrich, USA).
[0045] 1.2 Experimental animals
[0046] C57BL / 6 female mice (8 weeks) weighing 20±2 g were provided by the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine, animal qualification certificate number: SYXK (Shanghai) 2009-0069. Placed in a conventional feeding environment, free access to food and water.
[0047] 2. Experimental method
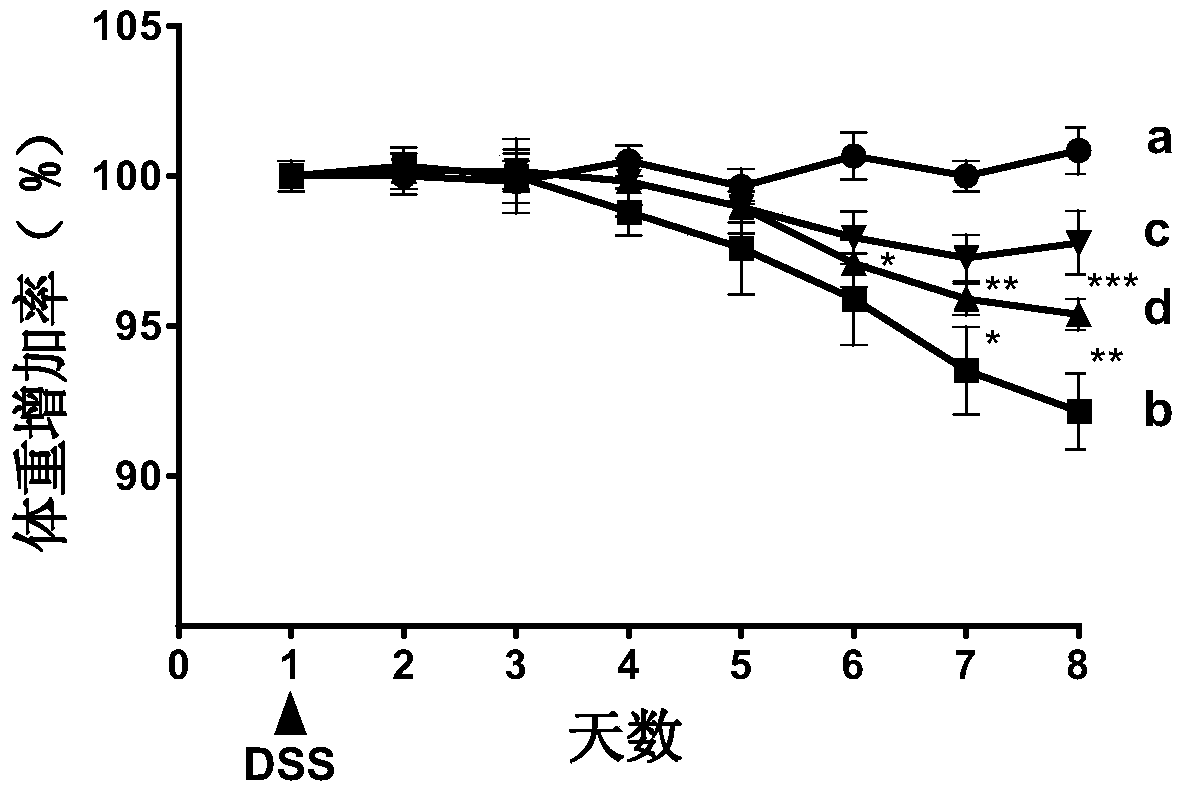
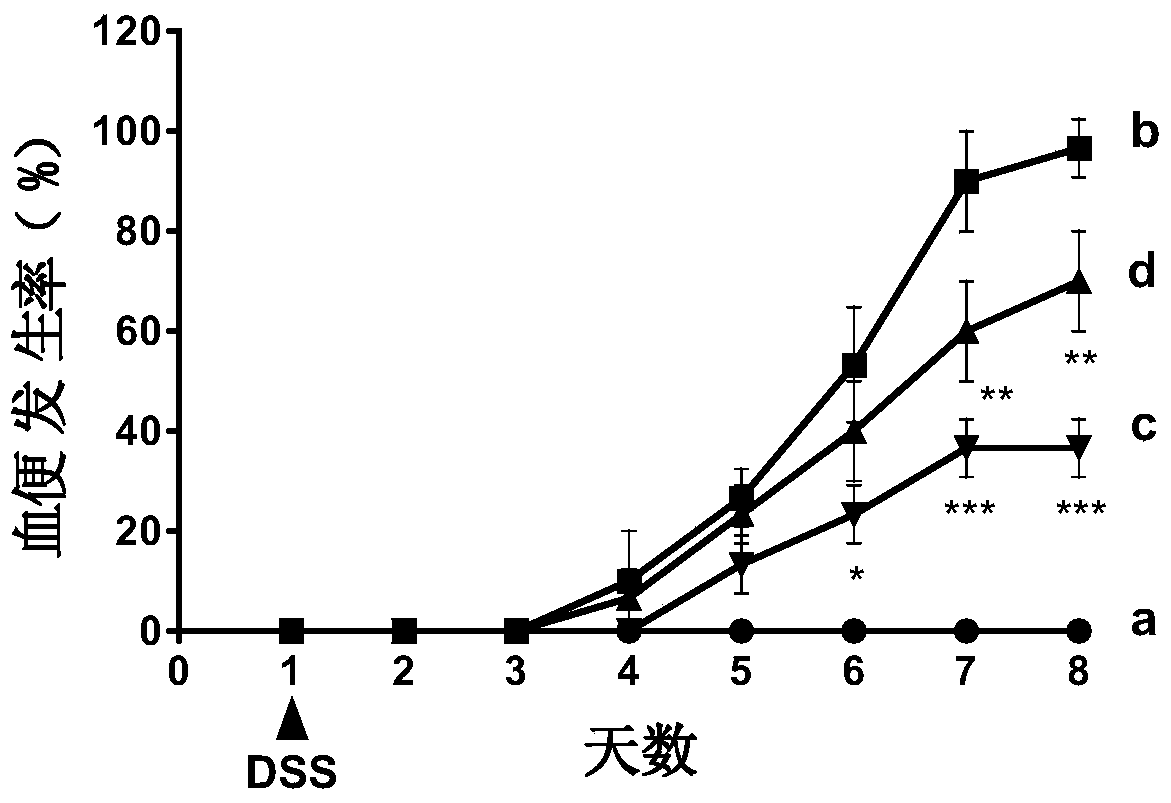
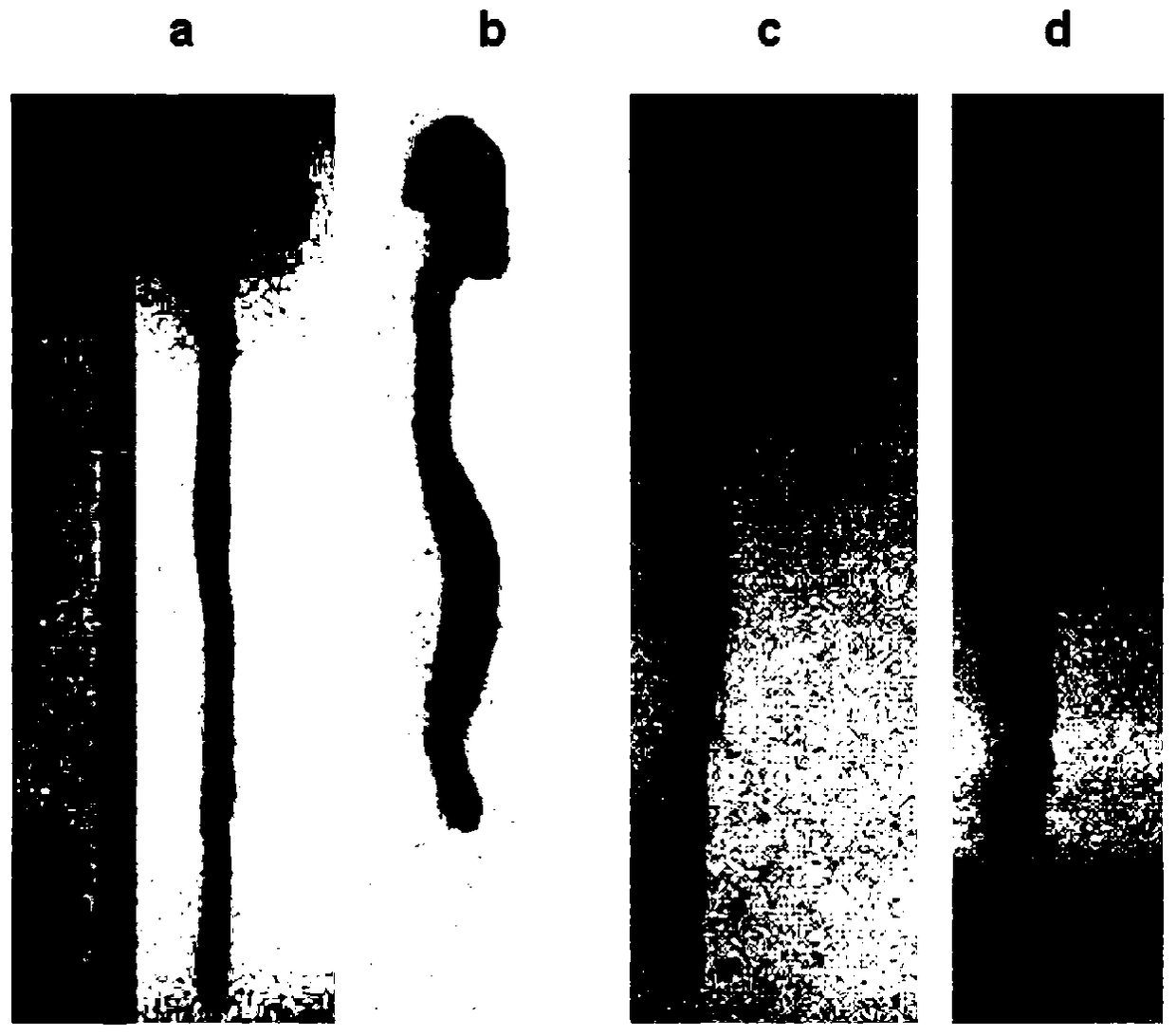
[0048] 2.1 Establishment of ulcerative colitis model
[0049] C57BL / 6 female mice (20±2g) with uniform body weight were selected, and the internationally accepted method for modeling ulcerative colitis (Gastroenterology2002, 123:256-70; PLoS...
Embodiment 2
[0073] The preparation of embodiment 2 tablet
[0074] Utilize conventional technique, mix following component, then directly compress tablet, prepare the pharmaceutical composition of tablet form, its formula is as follows:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com