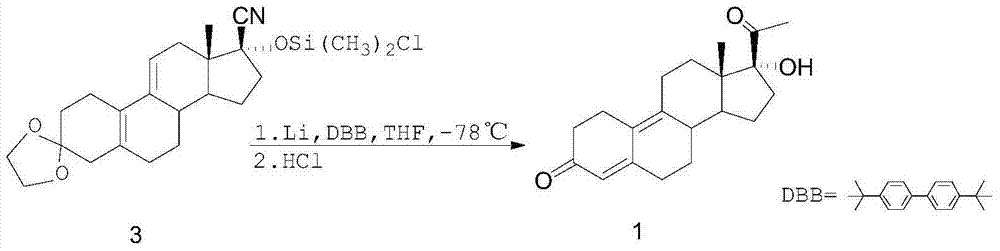
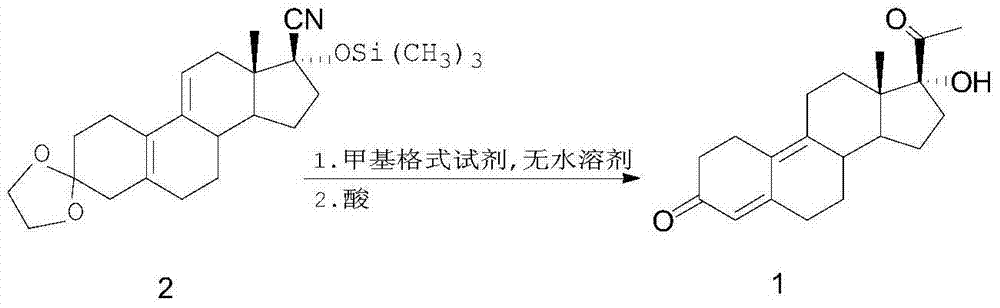
17alpha-hydroxy-19-norpregn-4,9-diene-3,20-dione preparation process
A preparation process, a pregnant-method, which is applied in the field of pharmaceutical preparation, can solve the problems of low cost and achieve the effects of easy operation, easy amplification and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 500ml four-necked round-bottomed flask, add 20g of compound 2, 150ml of a THF / toluene 1:1 mixed solution, and 64.6ml of a 3mol / L THF solution of methylmagnesium bromide. Stir continuously with a mechanical stirrer. The reaction solution was raised to 70°C, and after 96 hours of reaction, the TLC plate monitored the reaction raw materials to basically react completely, put the reaction solution into an ice-water bath to cool it to 0°C and slowly add 100ml of saturated aqueous ammonium chloride solution dropwise, After the dropwise addition, it was stirred at room temperature for 2 hours, then poured into a separating funnel, extracted with toluene, the organic layer was collected, and 100 ml of trifluoroacetic acid and water were added to the organic layer to stir at room temperature for 2 hours. After 1 hour, the reaction solution was cooled to 0°C, slowly added dropwise with ammonia water to neutralize the pH value to 7, then the organic layer was collected by liqu...
Embodiment 2
[0025] In a 500ml four-necked round-bottomed flask, add 20g of compound 2, 150ml of a THF / toluene 1:1 mixed solution, and 64.6ml of a 3mol / L THF solution of methylmagnesium bromide. Stir continuously with a mechanical stirrer. The reaction solution was raised to 80°C, and after 57 hours of reaction, the TLC plate monitored the reaction raw materials to basically react completely, put the reaction solution into an ice-water bath to cool it to 0°C and slowly add 100ml of saturated aqueous ammonium chloride solution dropwise, After the dropwise addition, it was stirred at room temperature for 2 hours, then poured into a separating funnel, extracted with toluene, the organic layer was collected, and 100 ml of trifluoroacetic acid and water were added to the organic layer to stir at room temperature for 2 hours. After 1 hour, the reaction solution was cooled to 0°C, slowly added dropwise with ammonia water to neutralize the pH value to 7, then the organic layer was collected by liqu...
Embodiment 3
[0027] In a 500ml four-necked round-bottomed flask, add 20g of compound 2, 150m of a mixed solution of THF:toluene=1:1, and 96.9ml of a 3mol / L THF solution of methylmagnesium bromide, and use a mechanical stirrer to keep stirring. , the reaction solution was raised to 70°C, and after 20 hours of reaction, the TLC plate monitored the reaction raw materials to basically react completely, put the reaction solution into an ice-water bath to cool it to 0°C and slowly add 100ml of saturated ammonium chloride aqueous solution dropwise , after the dropwise addition, it was stirred at room temperature for 2 hours, then poured into a separatory funnel, extracted with toluene, the organic layer was collected, and then 100 ml of trifluoroacetic acid and water were added to the organic layer to stir at room temperature After 2 hours, the reaction solution was cooled to 0°C, slowly added dropwise with ammonia water to neutralize the pH value to 7, then the organic layer was collected by liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com