Application of niacinamide as effective component in preparation of medicine for treating hepatitis B
A technology of nicotinamide and active ingredients, applied in the field of drug research and development, can solve the problems of virus resistance and low proportion, and achieve the effect of inhibiting expression and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 MTS test
[0043] Nicotinamide (NAM) (Sigma-Aldrich, product number: N0636) was dissolved in PBS to prepare a 1M Nicotinamide solution.
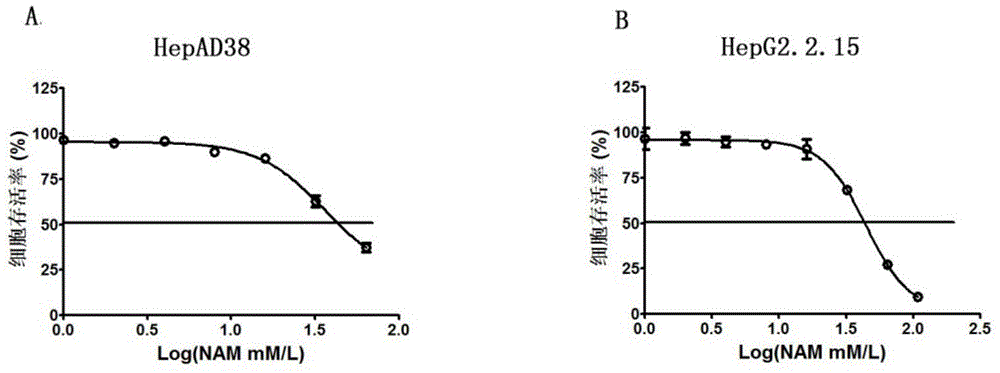
[0044] 1.5×10 4 HepAD38 cells or HepG2.2.15 cells were seeded in 96-well plates. After 24 hours, dilute 1M nicotinamide solution with MEM medium to 9 concentrations of 0mM, 1mM, 2mM, 4mM, 8mM, 16mM, 32mM, 64mM and 108mM Gradient, 3 replicate wells for each concentration, add 100 μl of the above solution to each well. After 72 hours, change to MEM medium. After continuing to culture for 48 hours, add 20 μl MTS reagent to each well, protect from light, incubate at 37° for 1 hour, detect OD value at 490 nm, calculate and draw the curve.
[0045] The result is as figure 1 Shown, nicotinamide is 36.38mM ( figure 1 A), in the cell HepG2.2.15 cell stably expressing HBV, CC50 is 44.44mM ( figure 1 B).
Embodiment 2
[0046] Example 2 The effect of nicotinamide on HBV replication in HBV replication cells HepAD38 and HepG2.2.15
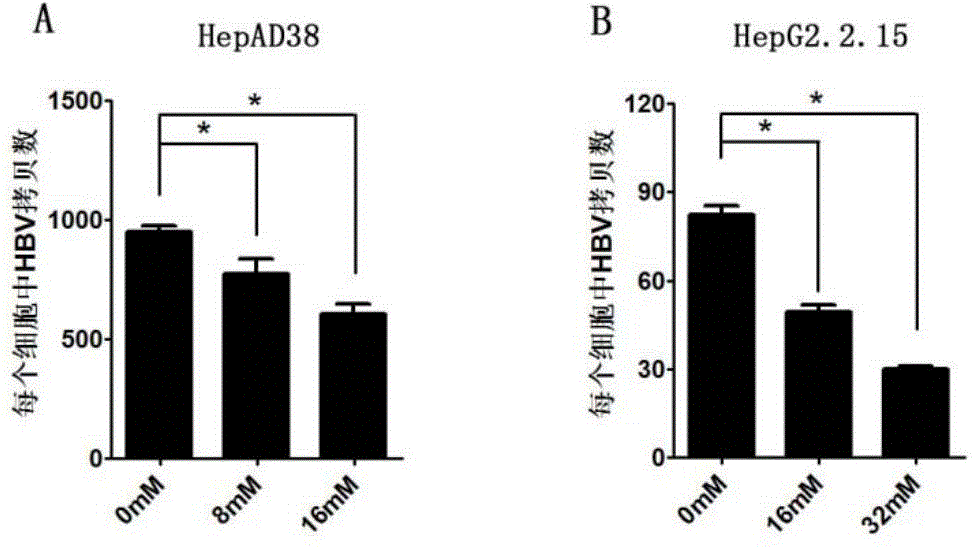
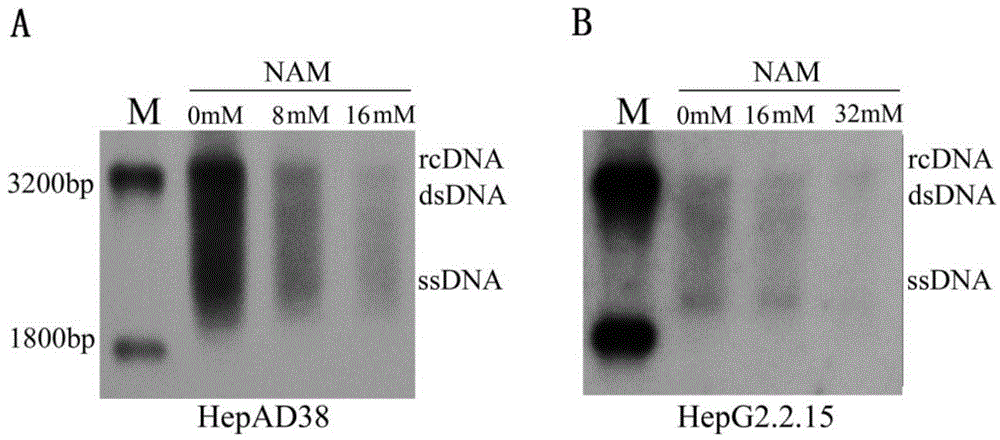
[0047] 25×10 4 HepAD38 or HepG2.2.15 cells were seeded in six-well plates. After 24 hours, prepare 0mM, 8mM, and 16mM nicotinamide solutions, and add 2ml of the above solutions to each well to treat HepAD38 cells (prepare 0mM, 16mM, and 32mM nicotinamide solutions, each well 2ml of the above solution to treat HepG2.2.15 cells). After 72 hours, change to MEM medium and continue culturing for 48 hours, digest and count the cells, extract HBV replication intermediates in the cells, extract cellular RNA, extract total cell protein, and collect the cell culture medium for HBsAg and HBeAg detection.
[0048] Real time PCR detection showed that nicotinamide significantly inhibited the formation of HBV replication intermediates. In HepAD38, compared with the control group, the HBV replication intermediates in the 8mM and 16mM nicotinamide groups were reduced to 70%, 60% (...
Embodiment 3
[0053] Example 3 Niacinamide can inhibit HBV replication in HBV transgenic mouse model
[0054] Fifteen female HBV transgenic mice (HBV-Tg C57BL / 6) aged 12-16 weeks and weighing 28.12±2.57 g were selected. Collect 100-300 μl of blood from the tail vein, incubate at 37° for 1 hour, centrifuge at 4000 rpm, 10 min, 4°C, transfer the serum to a clean EP tube (about 50-200 μl). HBV DNA in serum was extracted using Viral Genomic DNA / RNA Extraction Kit (TIANGEN, Cat. No. DP315), and the copy number of HBV DNA was detected. The HBsAg and HBeAg in the serum were detected by the hepatitis B virus surface antigen diagnostic kit (enzyme-linked immunoassay) and the hepatitis B virus e antigen diagnostic kit (enzyme-linked immunoassay) (Shanghai Kehua) respectively. Select 12 mice with high HBV DNA copy number and high HBsAg and HBeAg, divide the mice into 4 groups randomly, 3 mice in each group, and inject 0 μg / g and 10 μg / g prepared by sodium chloride respectively through the tail vein, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com