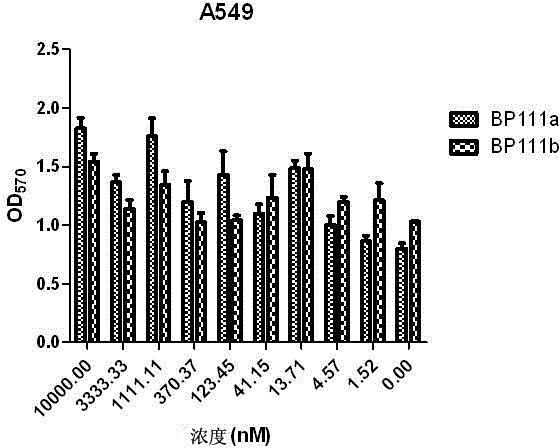
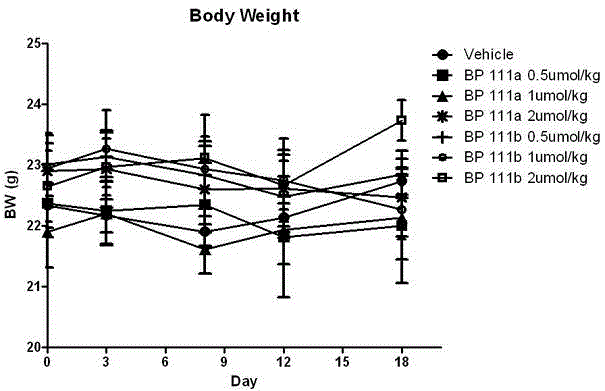
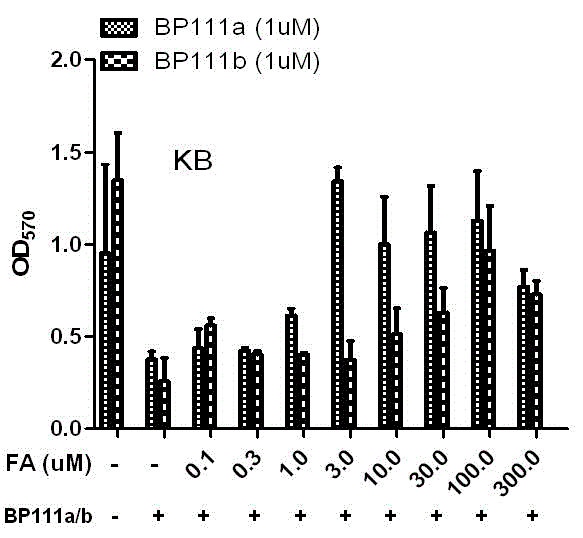
Folate receptor binding ligand-drug conjugate
A folic acid and drug technology, applied in the field of folic acid receptor binding ligand-drug conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1 Preparation of compound III
[0206] (III)
[0207] According to the conventional steps of the present invention, according to scheme 1, add 1000mg 2Cl-Trt Resin (1.5eq) to a 50ml reaction bottle, then add 234mg Fmoc-Cys(Trt)-OH (1.0eq), 8ml Chloromethane (DCM), 640μL DIEA (2.0eq), completely dissolved, reacted for 50min, added 8ml DCM, 1ml MeOH, 1ml DIEA, reacted for 20min, transferred to a self-made solid-phase synthesis reaction column, added 10ml 20% of Piperidine DMF solution, reacted for 20min, after washing with DMF, the ninhydrin test was positive, take 709mg Fmoc-Lys (Fmoc)-OH (3.0eqiv, 178mg HOBT (3.3eqiv), dissolve in 6ml DMF, add 230μL DIC (3.3eq) and mix After adding to the reaction column, after reacting for 1H, ninhydrin is monitored, and after washing with DMF, add 20% piperidine DMF solution. Repeat the above steps, sequentially condense Fmoc-Asp(OtBu)-OH, Fmoc-Asp(OtBu)-OH, Fmoc -Arg(Pbf)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Glu-OtBu, N 10 -TFA-...
Embodiment 2
[0208] Example 2 Preparation of compound 33
[0209]
[0210] Add 10.0ml DCM, 1.0ml MeOC(O)SCl (1.0eq) to a 100ml three-neck flask and cool down to 0°C in an ice bath, add 0.76ml mercaptoethanol (1.0eq) dropwise, keep warm at 0°C for 30min, add 1.22g 2- Add mercaptopyridine (1.0eq), 16ml DCM dropwise into the reaction bottle, keep warm at 0°C for 1h, then rise to room temperature for 1h, then TLC raw material reaction is complete, post-treatment: concentrate to about 16ml, filter with suction, the filtrate is washed with DCM to get light yellow Slight odor solid 2.0g.
[0211] Add 3.0ml DCM and 0.46g diphosgene (0.55eq) to a 100ml three-neck flask, cool down to 0°C in an ice bath, dissolve 1.0g compound 33-2 (1.1eq) in 13ml DCM, and add 0.45g Triethylamine (1.0eq) was dissolved and added dropwise to the reaction flask. During the dropwise addition, the temperature was controlled below 0°C. After rising to room temperature for 1 hour, the TLC reaction of the raw material...
Embodiment 3
[0213] Example 3 Preparation of compound 15-3
[0214]
[0215] Add 1.5g of vinblastine, 5ml of anhydrous methanol and 5ml of anhydrous hydrazine into a 100ml three-necked flask, heat up to about 60°C, react for 24 hours and then detect the reaction progress by TLC. Washed 3 times with water and 3 times with saturated brine, dried over anhydrous sodium sulfate and concentrated to obtain 1.1 g of white solid, namely compound 15-2.
[0216] Add 0.98g (1.0eq) of compound 15-2, 0.67g (1.5eq) of compound 33, and 10g of DCM into a 100ml three-necked flask, stir and dissolve, then add 0.44ml of triethylamine (2.5eq) dropwise to the reaction flask, and keep at room temperature for 2h After the reaction is complete.
[0217] Post-treatment: add 50ml of DCM, wash with water 3 times, wash with saturated brine 3 times, dry over anhydrous sodium sulfate, concentrate to obtain 1.2g of crude product, and obtain 750mg of pure product of 15-3 after column chromatography. MS[M] + : 95...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com