Plectasin mutant and its gene, preparation method and use
A technology of plectasin and mutants, applied in the field of bioengineering, can solve the problem of low antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Error-prone PCR amplification and construction of mutation library
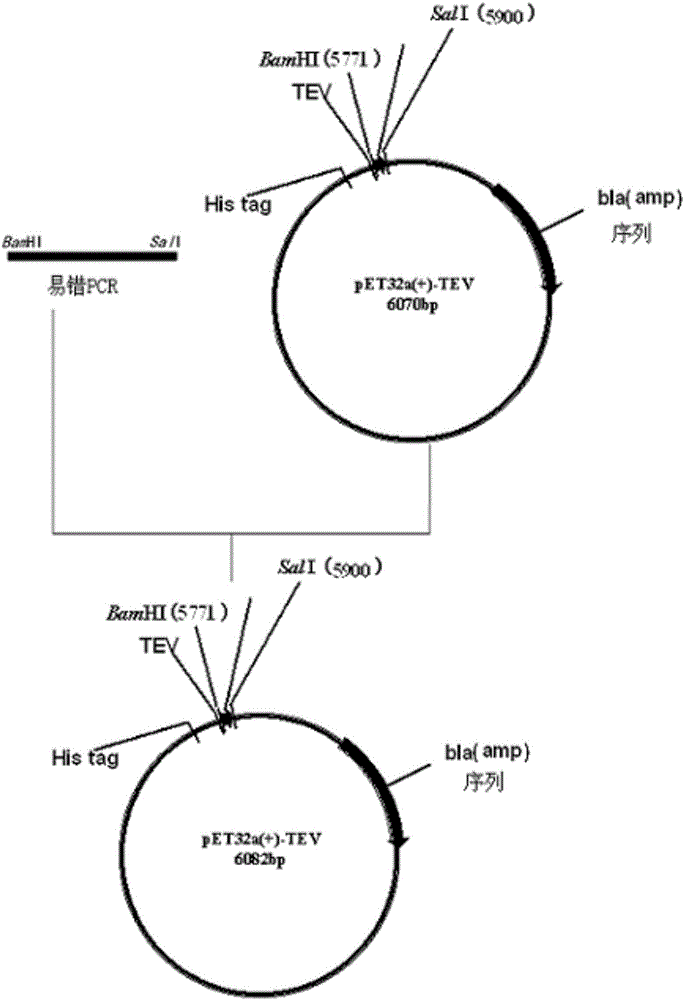
[0058] The present invention utilizes designed primers and plasmid pYG330 as a template (for the construction method of plasmid pYG330, please refer to the published Chinese patent application: 103374579A), explores error-prone PCR conditions, determines the optimal conditions for error-prone PCR, and constructs a mutant library. Since Mg2+ can stabilize non-complementary base pairs during the PCR process; Mn2+ can enhance the specificity of the polymerase to the template, thus adjusting the concentrations of the two ions can obtain diverse libraries with different mutation frequencies. In addition, lowering the annealing temperature, adopting a PCR amplification program with no hot start, no extension after PCR, and increased cycle times can also increase the mismatch rate. Gained error-prone PCR product is connected with Escherichia coli expression vector pET-32a (+)-TEV, the expression pla...
Embodiment 2
[0066] Screening and Identification of Example 2 Positive Clones
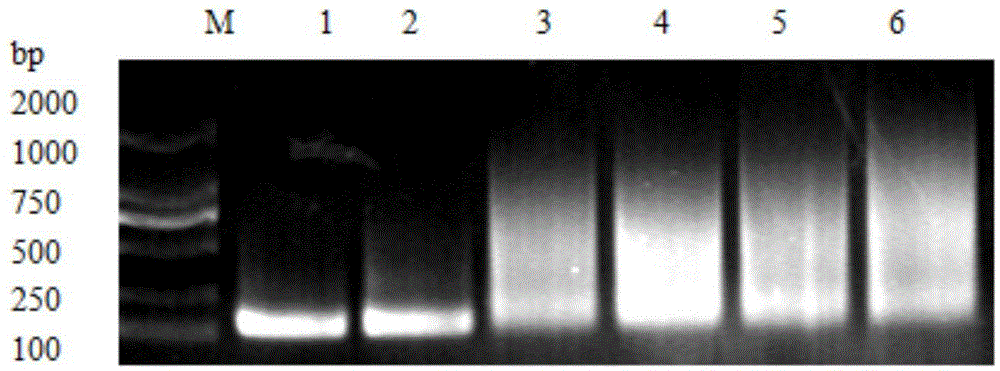
[0067] On the 6 plates obtained in Example 1, 20 monoclonal strains were randomly selected, cultivated at 37° C. for 12 hours, and the results of bacterial liquid PCR verification were as follows: Figure 5 As shown, where lane M is Marker; lanes 1 to 7 are the PCR identification results of different colonies. The obtained clones were sequenced by Shanghai Sangon. The sequencing results showed that when the concentration of Mg2+ in the PCR buffer was 15mM, 18mM and 20mM, base mutations occurred in the obtained PCR, and the results are shown in Table 1.
[0068] Table 1 Different Mg 2+ Sequencing results of error-prone PCR-positive transformants at concentrations
[0069]
[0070] Wherein when the PCR buffer Mg 2+ When the concentration was 15mM, 2 of the transformants obtained had base mutations, one was a transversion (the 92nd base was mutated from C to A), and the other was a nonsense mutation; Mg 2+...
Embodiment 3M-1
[0077] Example 3 Induced expression and preliminary separation and purification of M-1, M-4, M-6, M-7 and plectasin
[0078] The plectasin-expressing strain was used as a control strain (for the preparation method of the plectasin-expressing strain, please refer to the published Chinese patent application: 103374579A), the expression vector of this strain is pYG330, and the expression host is E.coli.BL21( DE3). Inoculate 2.5ml of M-1, M-4, M-6, M-7 and the overnight culture of the above-mentioned plectasomycin expression strains into 250ml of LB medium (containing 100μg / ml Amp), shake culture at 37°C for 2.5h to The OD600 was 0.4-0.6, and 0.8 mM IPTG was added to induce expression for 3 hours, and then the bacteria were collected. The induced culture was centrifuged (8000rpm×10min) to obtain the bacteria. Resuspended with 10ml NTA-0, placed in an ice bath and ultrasonically disrupted the wall for 10min (500W, working for 5s, resting for 5s). The cell wall solution was centr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com