Stable pharmaceutical composition comprising solifenacin, and method for preparing the same
一种索利那新、混合物的技术,应用在包括索利那新的稳定药物组合物及其制备领域,能够解决分解、湿法不稳定性等问题,达到无重量偏差、杰出含量均匀性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Mill mixtures 1 and 2 were mixed in a 1 kg mixer (erweka AR402 / cubic mixer) at 200 rpm for 15 minutes, and the resulting mixture was lubricated and mixed with 7.5 g of magnesium stearate, filtered through a 30-mesh sieve, and then filtered using a single A tablet press (erweka AR401) forms round tablets. The respective component contents of the tablets thus obtained are shown in Table 1 below.
[0047] 【Table 1】
[0048] mixed purpose
[0049] Examples 2 to 7 and Comparative Example 1
Embodiment 2 to 7 and comparative Embodiment 1
[0051] Regarding the preparation of tablets with the compositions of Examples 2 to 7 and Comparative Example 1, and 16.65 g of solifenacin succinate, butylated hydroxytoluene (varies from 0.25 g to 0.5 g, depending on Example 2 to 7), 100.0 g of lactose hydrate and 50.0 g of microcrystalline cellulose were mixed by milling, and then filtered through a 30-mesh sieve (grinding and mixing-1). Subsequently, 100.0 g of microcrystalline cellulose and lactose hydrate (ranging from 212.35 g to 212.85 g, depending on Examples 2 to 7 and Comparative Example 1) were then filtered through a 30 mesh screen (Grind Mix-2).
[0052] Mill mixtures 1 and 2 were mixed in a 1 kg mixer (erweka AR402 / cubic mixer) at 200 rpm for 15 minutes, and the resulting mixture was lubricated and mixed with 7.5 g of magnesium stearate, filtered through a 30-mesh sieve, and then filtered using a single A tablet press (erweka AR401) forms round tablets. The core tablets thus completed are used for (yellow, opa...
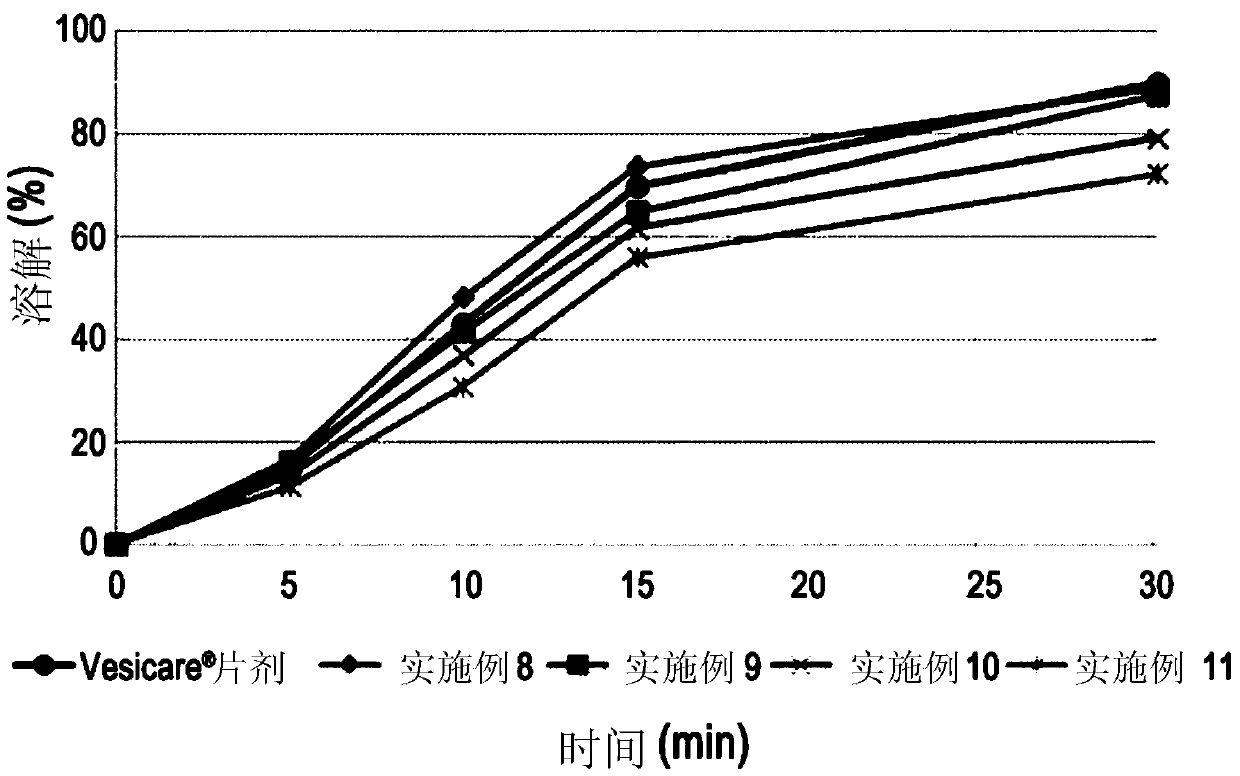
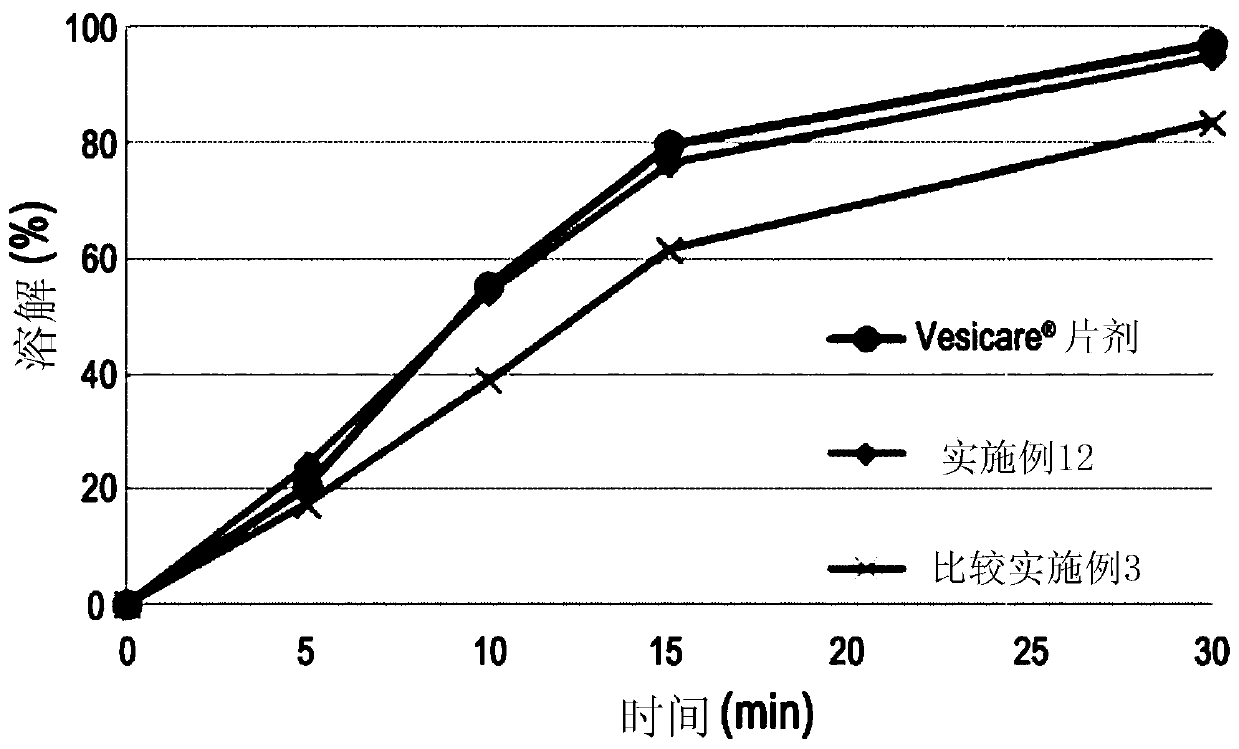
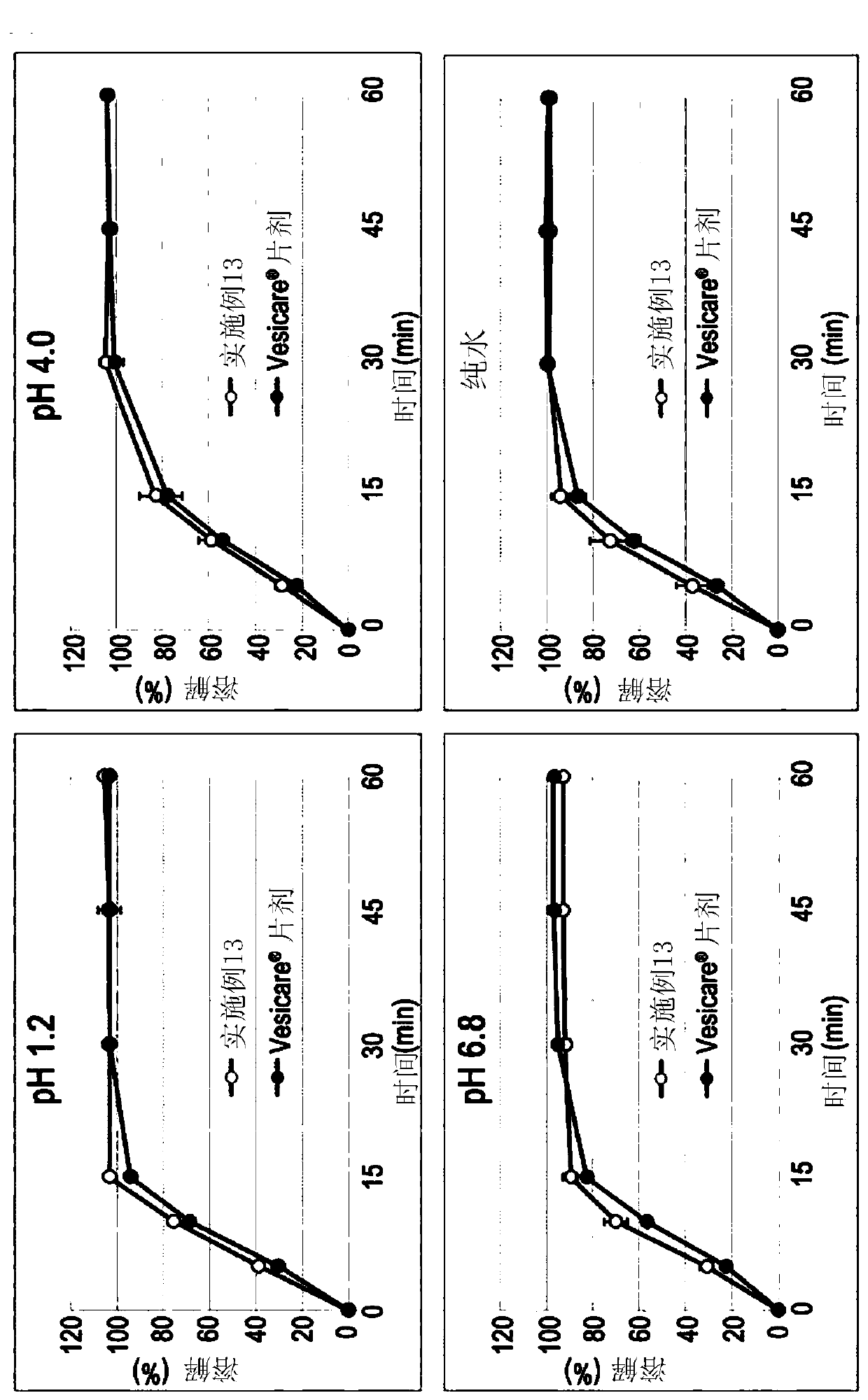
Embodiment 8 to 12 and comparative Embodiment 2 and 3
[0058] 16.65 g of solifenacin succinate, 0.5 g of butylated hydroxytoluene, low-substituted hydroxypropyl cellulose (ranging from 0.0 g to 150.0 g, depending on each combination of Examples 8 to 11 and Comparative Example 2 ) and 95.0 g of isomalt were mixed by grinding and filtered through a 30-mesh sieve (Grind Mix-1).
[0059] Subsequently, 10.0 g of hydrophobic colloidal silicon dioxide and lactose hydrate (ranging from 207.35 g to 357.35 g, depending on Examples 8 to 11 and Comparative Example 2) were mixed and filtered through a 30-mesh sieve (Grind Mix-2 ).
[0060] Mill mixtures 1 and 2 were mixed in a 1 kg mixer (erweka AR402 / cubic mixer) at 200 rpm for 15 minutes, and the resulting mixture was lubricated and mixed with 7.5 g of magnesium stearate, filtered through a 30-mesh sieve, and then filtered using a single A tablet press (erweka AR401) forms round tablets. The core tablets thus completed are used for (yellow, opadry03B52293) coating. The respective compon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com