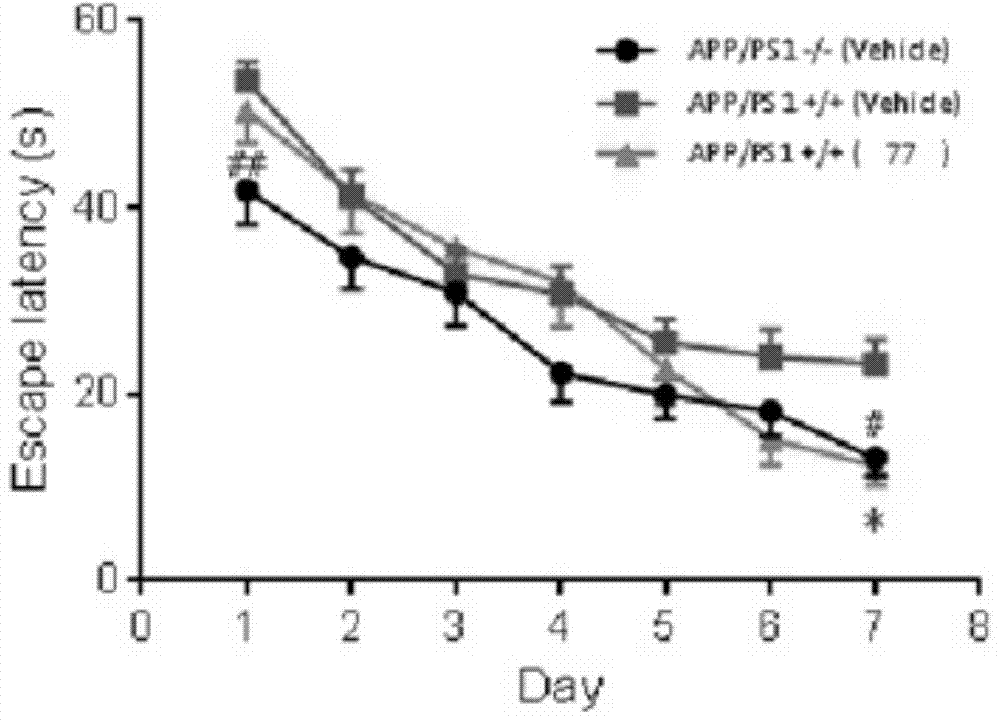
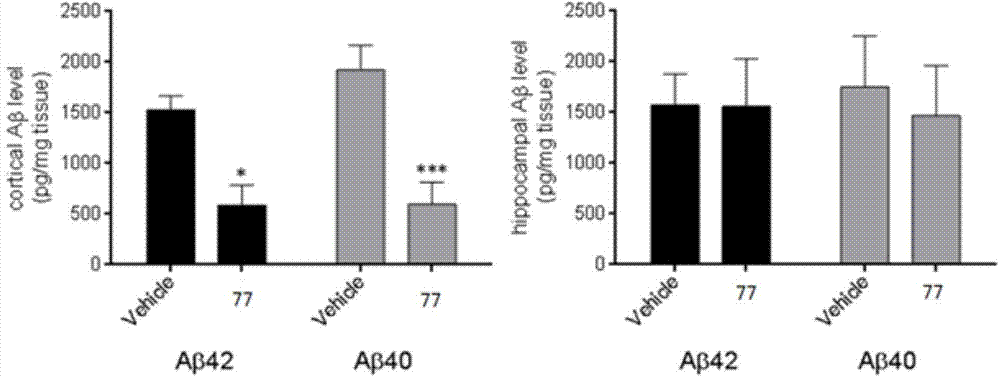
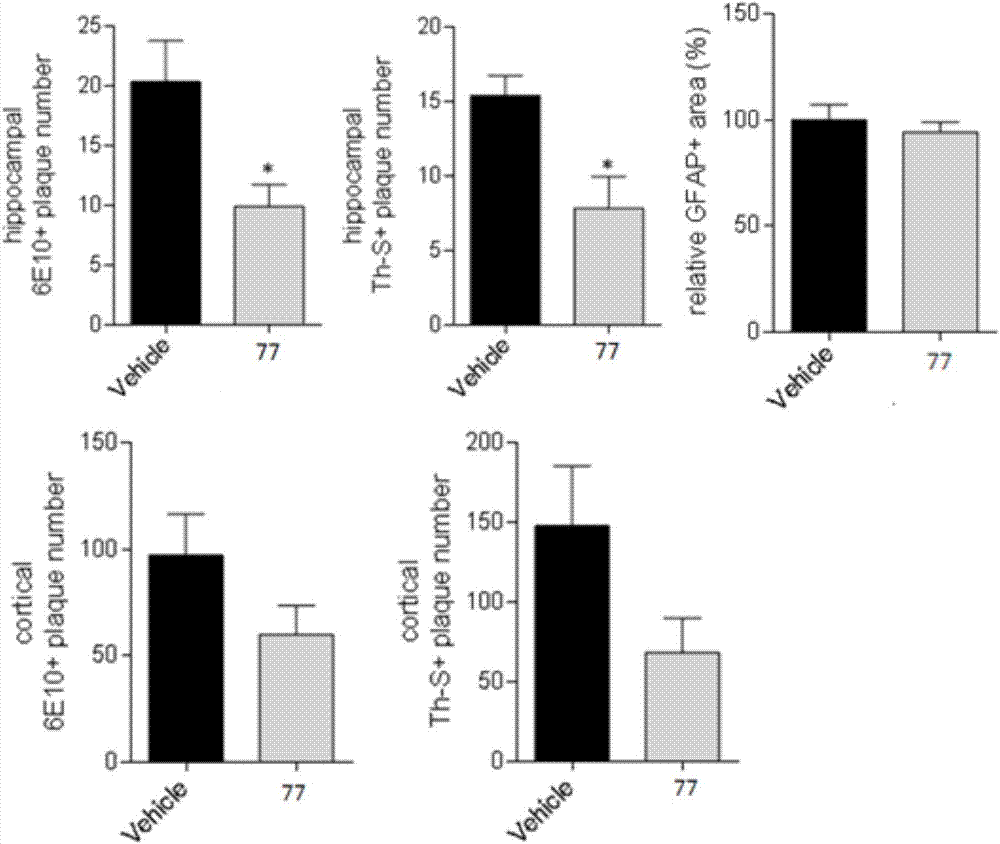
Compound used as DOR receptor antagonist
A technology of compounds and receptors, applied in the field of compounds, can solve problems that cannot greatly improve memory or effectively cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Compound 1
[0105] N,N-Diethyl-4-(2-(((2-(trifluoromethyl)benzyl)amino)methyl)-1H-indene-3-position)benzamide
[0106]
[0107] Intermediate 1-1: 3-Chloro-1H-indene-2-carbaldehyde
[0108]
[0109] Cool 4mL of phosphorus oxychloride to 0°C, add DMF (800mg, 11mmol), stir for 30min, add 1-indanone (1.32g, 10mmol), stir the reaction system at room temperature for 2h, then heat to 70°C to clarify. After cooling to room temperature, the reactant was poured into 50 g of crushed ice, extracted with EA (50 mL*3), and the organic layer was washed with water (100 mL*2), saturated sodium bicarbonate (100 mL), and saturated sodium chloride (100 mL). Concentration gave a crude product, which was purified by column chromatography (EA:PE=1:30) to give Intermediate 1-1 as a pale yellow solid (750 mg, 42%).
[0110] Intermediate 1-2: N,N-Diethyl-4-(2-formaldehyde-1H-indene-3-position)benzamide
[0111]
[0112]Compound 1-1 (400mg, 2.25mmol), boroester 1-2 (1.10g, 3.63mmol)...
Embodiment 2
[0118] Compound 2
[0119] N,N-Diethyl-4-(5-hydroxy-2-(((2-(trifluoromethyl)benzyl)amino)methyl)-1H-indene-3-position)benzamide
[0120]
[0121] Intermediate 2-2: 3-Chloro-5-methoxy-1H-indene-2-carbaldehyde
[0122]
[0123] Add 1ml of phosphorus oxychloride to a 25ml single-necked bottle, seal it tightly, and place it in an ice-water bath to cool to 0°C. Inject DMF (216mg, 2.96mmol) with a syringe and stir for 20min. 2-1 (400mg, 2.47mmol) was added to the above system and replaced with nitrogen 3 times. After stirring at room temperature for 3 hours, TLC showed that a small amount of compound 1 was not converted. Pour the system into 20ml of ice water to quench, add 50ml of EA, separate the layers, extract the aqueous layer with 50ml of EA, combine the organic layers, wash once with saturated NaCl, and then wash with anhydrous sulfuric acid Sodium-dried, dried, and purified by column chromatography, intermediate 2-2 (150 mg, 29.2%) was obtained.
[0124] Intermedia...
Embodiment 3
[0135] Compound 3
[0136] N,N-Diethyl-4-(2-(((2-fluorophenethyl)amino)methyl)-5-hydroxy-1H-indene-3-position)benzamide
[0137]
[0138] Compound 3 can be prepared by replacing 2-trifluoromethylphenethylamine with 2-fluorophenethylamine according to the method for preparing compound 2.
[0139] 1 H NMR (400MHz, CDCl 3 )δ7.39(d, J=8.0Hz, 2H), 7.24-7.11(m, 5H), 7.04-6.96(m, 2H), 6.67(dd, J1=8.0Hz, J2=2.0Hz, 1H), 6.61(d,J=2.0Hz,1H),3.67(s,2H),3.57(m,2H),3.47(s,2H),3.31(m,2H),2.87-2.81(m,4H),1.27 -1.23(m,3H),1.19-1.14(m,3H); ESI-MS m / z 459.5(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com