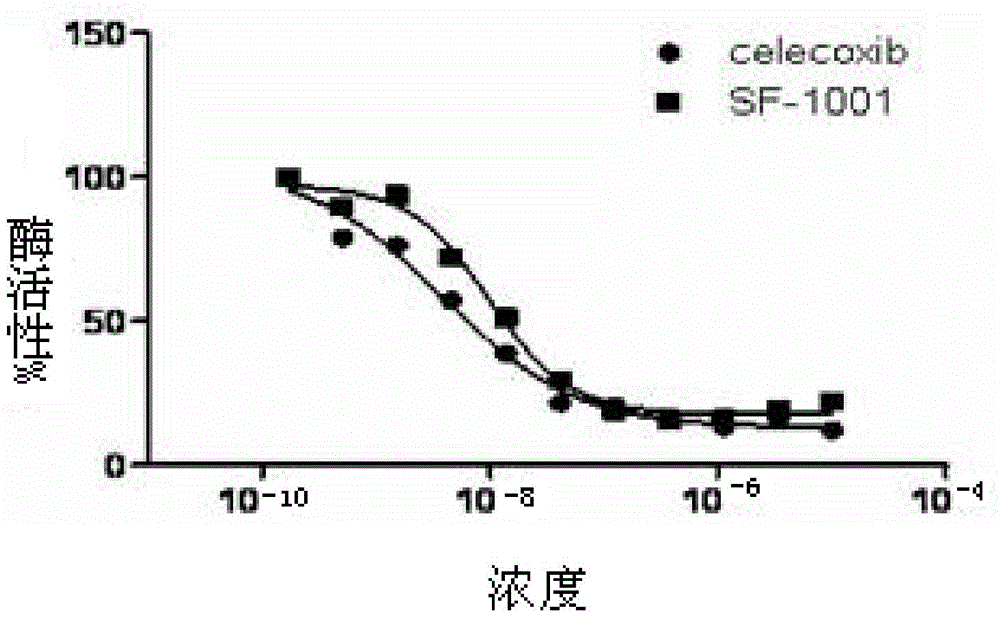
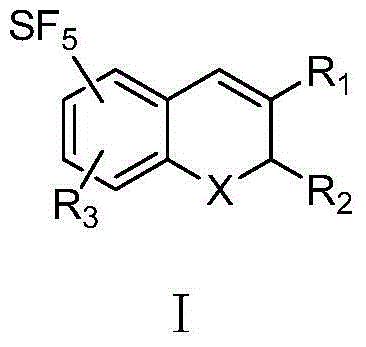
Sulfur pentafluoride-substituted benzopyran compound and use thereof
A technology of sulfur pentafluoride and benzopyran, which is applied in the field of sulfur pentafluoride substituted benzopyran compounds, can solve the problems of undiscovered alternative drugs, withdrawal, cardiovascular risk, etc., and achieve excellent inflammation and pain, Reduce the possibility of high blood pressure, reduce the effect of abnormal pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Preparation of 6-sulfur-pentafluoride-2-trifluoromethyl-2H-benzopyran-3-carboxylic acid.
[0102]
[0103] Prepare according to the above reaction scheme, comprising the following steps:
[0104] 1. Synthesis of Compound 2
[0105] Dissolve compound 1 (1g, 4.5mmol) in trifluoroacetic acid (20mL), then slowly add hexamethylenetetramine (0.88g, 6.3mmol) to the solution, keep the temperature at 80°C for 4h, stop heating and cool to At room temperature, 10 mL of water was added and stirred for 0.5 h. After the reaction was completed, saturated sodium bicarbonate solution was added, extracted with ethyl acetate, and the extracted organic phase was washed with brine, dried, rotary evaporated under reduced pressure, and compound 2 (0.6 g, yield 52%) was obtained by column chromatography.
[0106] The characterization data of this compound 2 are:
[0107] 1H NMR (400MHz, d-CDCl3), d11.32 (s, 1H), 9.95 (s, 1H), 8.011 (s, 1H), 7.93-7.90 (d, 1H), 6.88-6.86 (d, 1H).
[0108]...
Embodiment 2
[0117] (S) Preparation of 6-sulfur-pentafluoride-8-bromo-2-trifluoromethyl-2H-chromene-3-carboxylic acid.
[0118]
[0119] Prepare according to the above reaction scheme, comprising the following steps:
[0120] 1. Synthesis of Compound 5
[0121] Compound 1 (2g, 9.2mmol) and anhydrous potassium carbonate (3.8g, 27.6mmol) were added to DMF (30ml), and methyl iodide (1.32g, 9.2mmol) was added dropwise under ice cooling, and stirred overnight at room temperature. After the reaction, water was added and extracted with ethyl acetate. The extracted organic phase was washed with saturated brine, dried, and rotary evaporated under reduced pressure. Compound 5 (1 g, yield 93%) was obtained by column chromatography.
[0122] The characterization data of this compound 5 are:
[0123] 1H NMR (400MHz, d-CDCl3), d7.72-7.69 (d, 2H), 6.94-6.92 (d, 2H), 3.3.87 (s, 3H).
[0124] 2. Synthesis of Compound 6
[0125] Compound 5 (2g, 8.56mmol) was dissolved in acetic acid (30ml), and liqui...
Embodiment 3
[0149] (R) Preparation of 6-sulfurpentafluoride-2-trifluoromethyl-2H-chromene-3-carboxylic acid.
[0150]
[0151] According to the above reaction scheme, the above compound 12 was prepared with reference to the method of Example 2. Wherein, the catalyst used in the synthesis of compound 11 is (2R)-2-[diphenyl[(trimethylsilyl)oxy]methyl]-pyrrolidine.
[0152] The characterization data of this product (R) 6-sulfur pentafluoride-2-trifluoromethyl-2H-chromene-3-carboxylic acid is:
[0153] 1HNMR(400MHz,d-CDCl3),d7.85(s,1H),7.77-7.75(d,1H),7.70(s,1H),7.10-7.08(d,1H),5.80-5.78(d,1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com