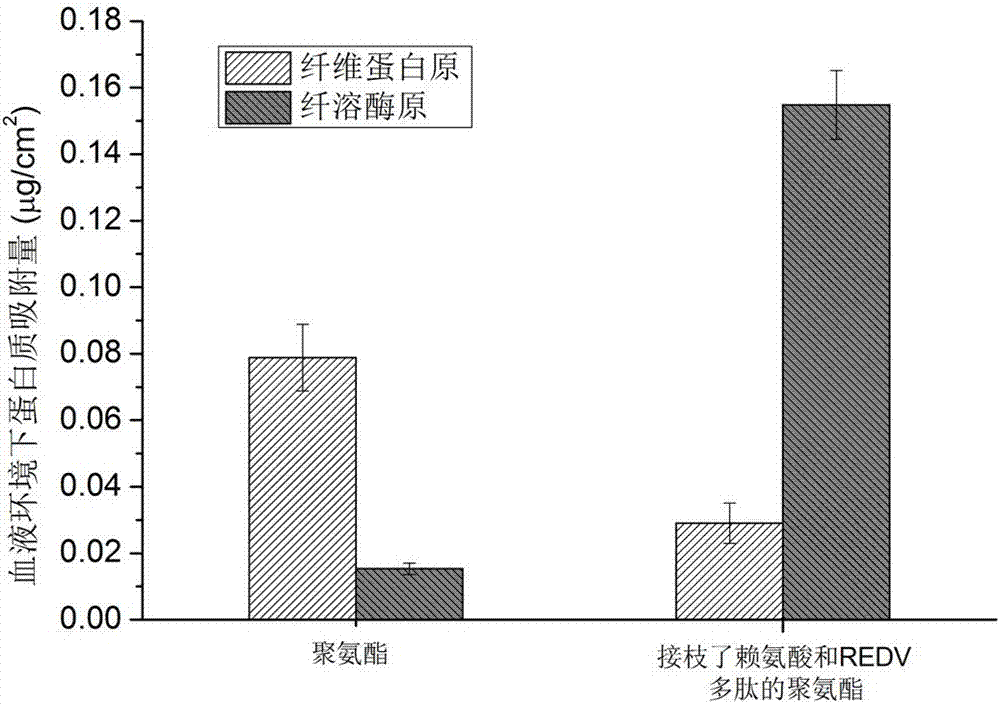
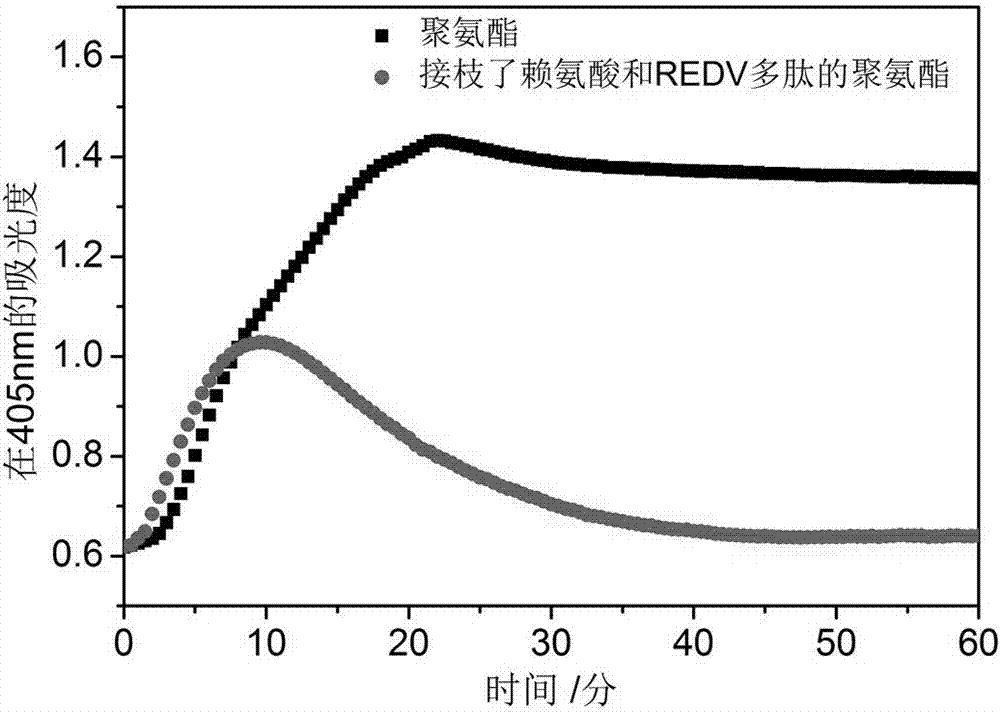
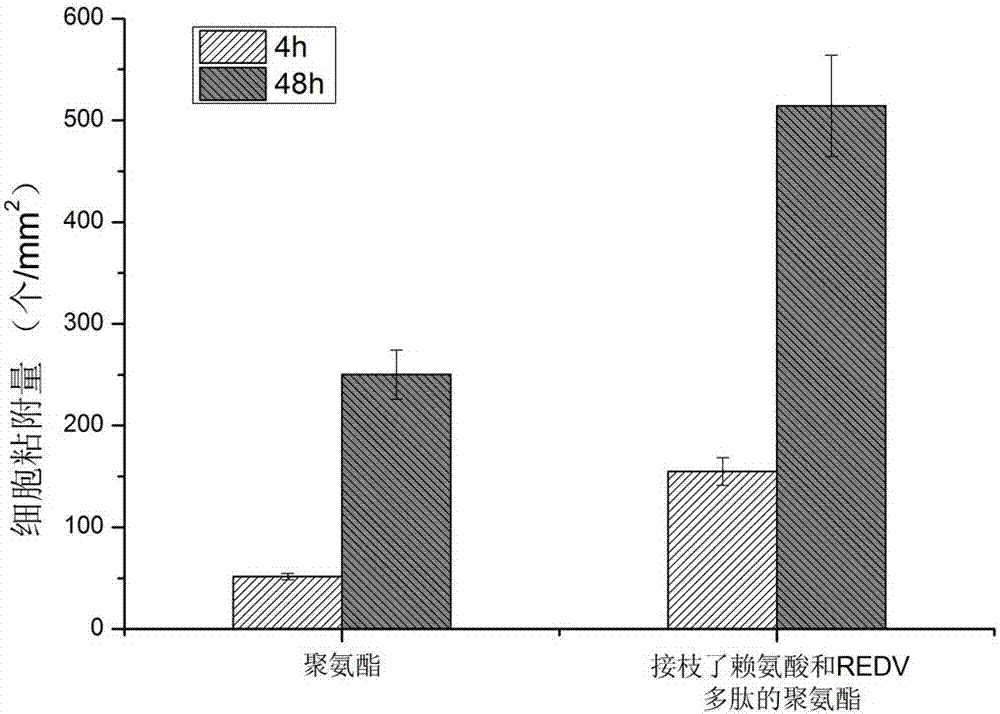
Polyurethane derivatives simulating human fibrinolytic system and vascular endothelial system, preparation method and related product preparation method
A technology of polyurethane derivatives and fibrinolytic systems, applied in prosthesis, transportation and packaging, anticoagulation treatment, etc., can solve problems such as restricting development, uncontrollable binding amount, harsh reaction conditions of bioactive molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] (c) Preparation of lysine-modified cyclodextrin (β-CD-(Lys) 7 )
[0070] Alkynylated lysine and azidated β-cyclodextrin were subjected to click reaction, followed by deprotection treatment, and finally lysine-modified cyclodextrin was prepared.
[0071] The preparation method described in the above step (a) is one or both of the following:
[0072] First, the hydroxyl group at the sixth position of β-cyclodextrin is replaced by bromine (β-CD-(Br) 7 ), followed by azidation with sodium azide;
[0073] First, replace the hydroxyl group at the sixth position of β-cyclodextrin with iodine (β-CD-(I) 7 ), followed by azidation with sodium azide.
[0074] 4-oxo-4-(prop-2-yn-1-oxy)butanoic anhydride (4-oxo-4-(prop-2-yn-1-yloxy)butanoic anhydride described in step (b) above ) solution and a mixed solution of N(ε)-Boc-L-lysine tert-butyl ester hydrochloride and triethylamine, the solvents of which are dichloromethane.
[0075] The method used for the deprotection described ...
specific Embodiment
[0079](1) Polyurethane surface graft copolymerization P(HEMA-co-AdaMA)
[0080] Put 0.98 g of potassium thiocyanate and 40 mL of anhydrous acetonitrile in a 100 mL reaction flask and stir to dissolve. Slowly drop 0.82 mL of acryloyl chloride into the reaction flask, and stir the reaction at room temperature for 12 hours. The precipitate was removed by filtration, and the filtrate was directly used in the next reaction. Put 60 polyurethane diaphragms (thickness 0.5 mm) with a diameter of about 6 mm in a reaction flask containing 30 mL of the above filtrate, add 0.75 g of triethylamine to the reaction flask, stir and react at 65 °C for 2-12 hours, and then place The polyurethane diaphragm is taken out, washed with acetonitrile and dried to obtain a polyurethane diaphragm with carbon-carbon double bonds on the surface. Place 0.074 g of azobisisobutyronitrile, 5.78 g of HEMA, 0.13 g of AdaMA, 40 mL of isopropanol, and 60 pieces of polyurethane diaphragms with carbon-carbon doubl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com