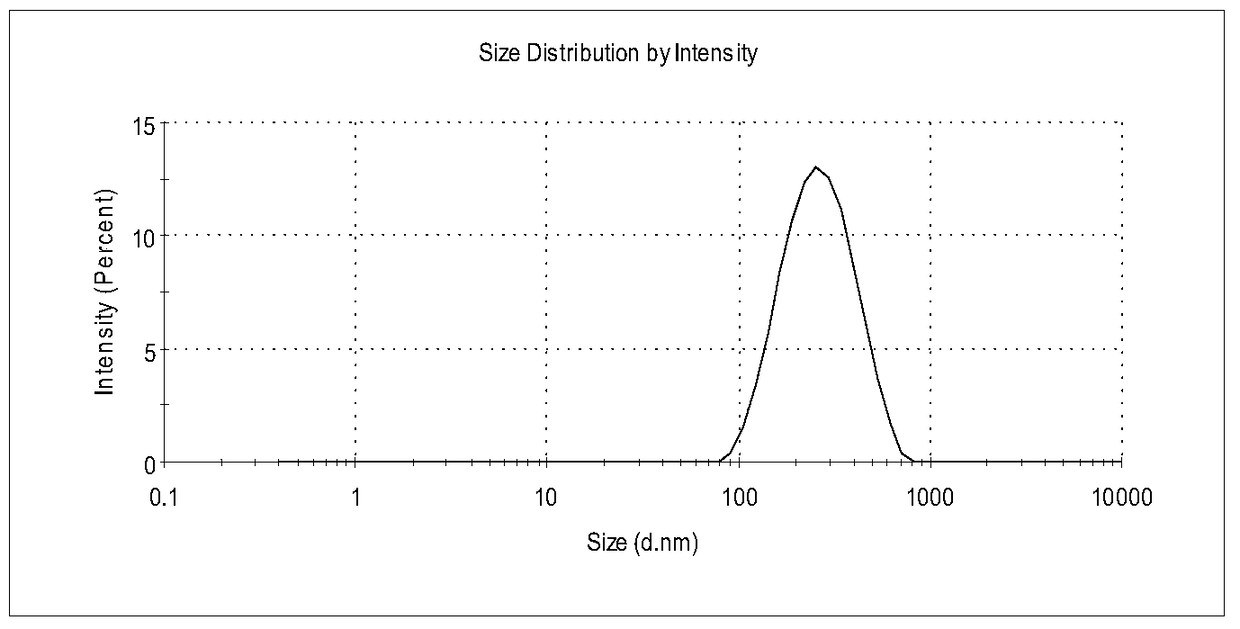
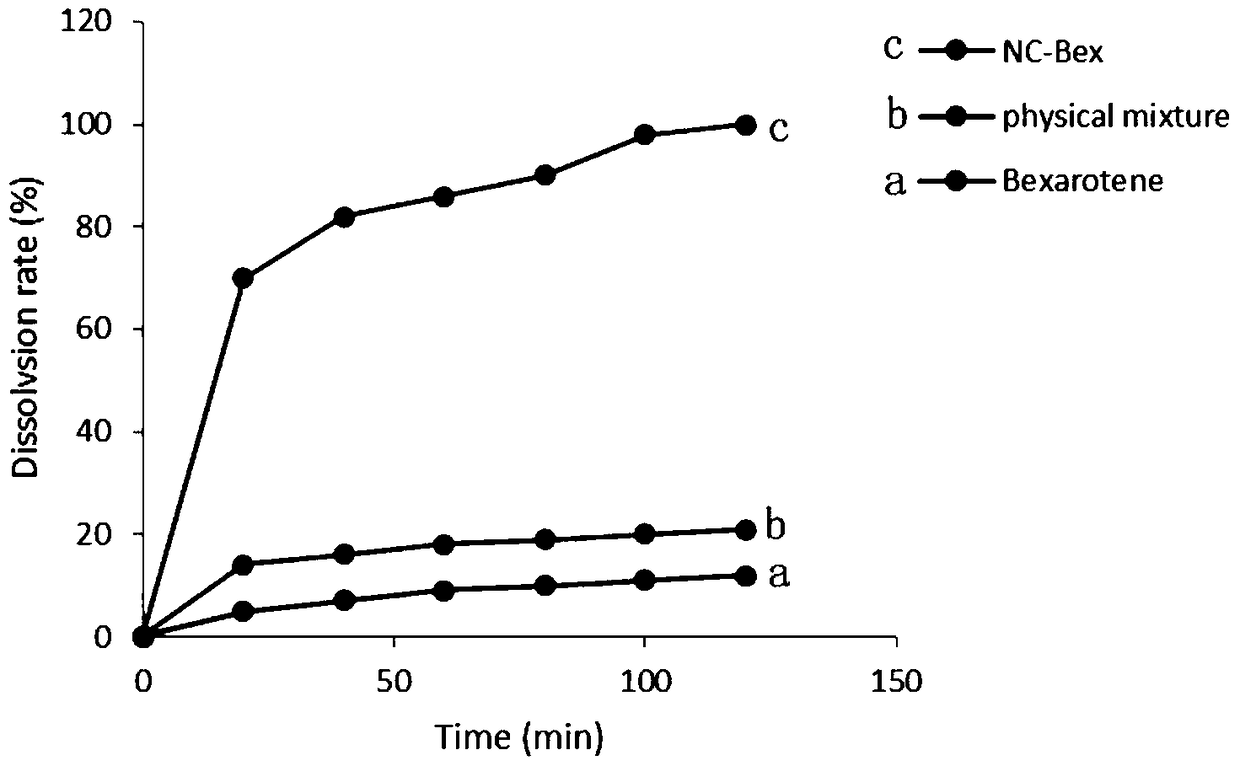
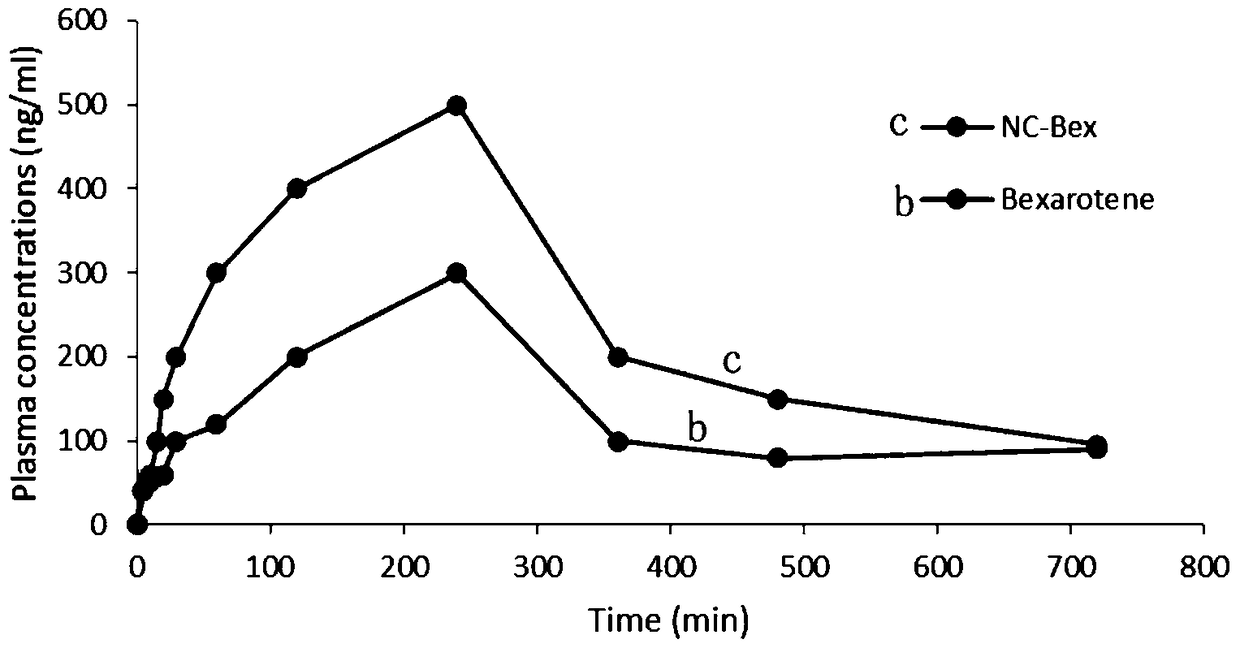
A kind of bexarotene nano-suspension
A nano-suspension, bexarotene technology, applied in emulsion delivery, medical preparations of non-active ingredients, anti-tumor drugs, etc., can solve the problems of poor dissolution, low bioavailability and difficulty in making common preparations Injection and other problems, to achieve the effect of improving bioavailability, simple preparation process, and uniform release in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Bexarotene nanosuspension
[0025] The prescription is composed of: 0.015g of bexarodene as the main drug, and 0.015g of polyvinylpyrrolidone as the surfactant.
[0026] The specific preparation method is as follows:
[0027] Weigh 0.015g of bexarodene, dissolve in 10ml of 0.1mol / L sodium hydroxide solution, then slowly add dropwise to 10ml of 0.1mol / L hydrochloric acid solution containing 0.015g of polyvinylpyrrolidone, and magnetically stir to obtain Milky suspension. Centrifuge the milky suspension obtained by magnetic stirring, take the precipitate, and add 20ml of distilled water to disperse. The dispersed solution was pulverized by an ultrasonic cell pulverizer at a power of 180 W for 10 minutes to obtain a bexarotene nanosuspension.
Embodiment 2
[0028] Example 2 Bexarotene nanosuspension
[0029] The prescription is composed of: 0.015g bexarotene as the main drug, and 0.0225g polyvinylpyrrolidone as the surfactant.
[0030] The specific preparation method is as follows:
[0031] Weigh 0.015g of bexarodene, dissolve it in 10ml of 0.1mol / L sodium hydroxide solution, then slowly add it dropwise to 10ml of 0.1mol / L hydrochloric acid solution containing 0.0225g of polyvinylpyrrolidone, and stir magnetically to obtain Milky suspension. Centrifuge the milky suspension obtained by magnetic stirring, take the precipitate, and add 20ml of distilled water to disperse. The dispersed solution was pulverized by an ultrasonic cell pulverizer at a power of 180 W for 10 minutes to obtain a bexarotene nanosuspension.
Embodiment 3
[0032] Example 3 Bexarotene nanosuspension
[0033] The prescription is composed of: 0.015g of bexarodene as the main drug, and 0.03g of polyvinylpyrrolidone as the surfactant.
[0034] The specific preparation method is as follows:
[0035] Weigh 0.015g of bexarodene, dissolve it in 10ml of 0.1mol / L sodium hydroxide solution, then slowly add it dropwise to 10ml of 0.1mol / L hydrochloric acid solution containing 0.03g of polyvinylpyrrolidone, and stir magnetically to obtain Milky suspension. Centrifuge the milky suspension obtained by magnetic stirring, take the precipitate, and add 20ml of distilled water to disperse. The dispersed solution was pulverized by an ultrasonic cell pulverizer at a power of 180 W for 10 minutes to obtain a bexarotene nanosuspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com