Metronidazole derivative and preparing method and application thereof
A technology of metronidazole and derivatives, applied in the field of medicinal chemistry, can solve the problems of affecting physiological functions, high price, side effects, etc., and achieve a broad-spectrum anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
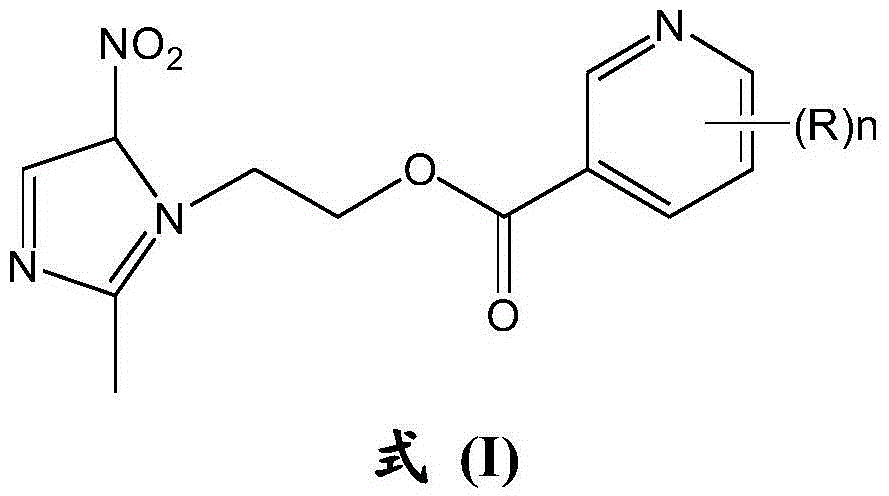
[0055] Synthesis of Class A metronidazole derivatives shown in embodiment 1 formula (I)
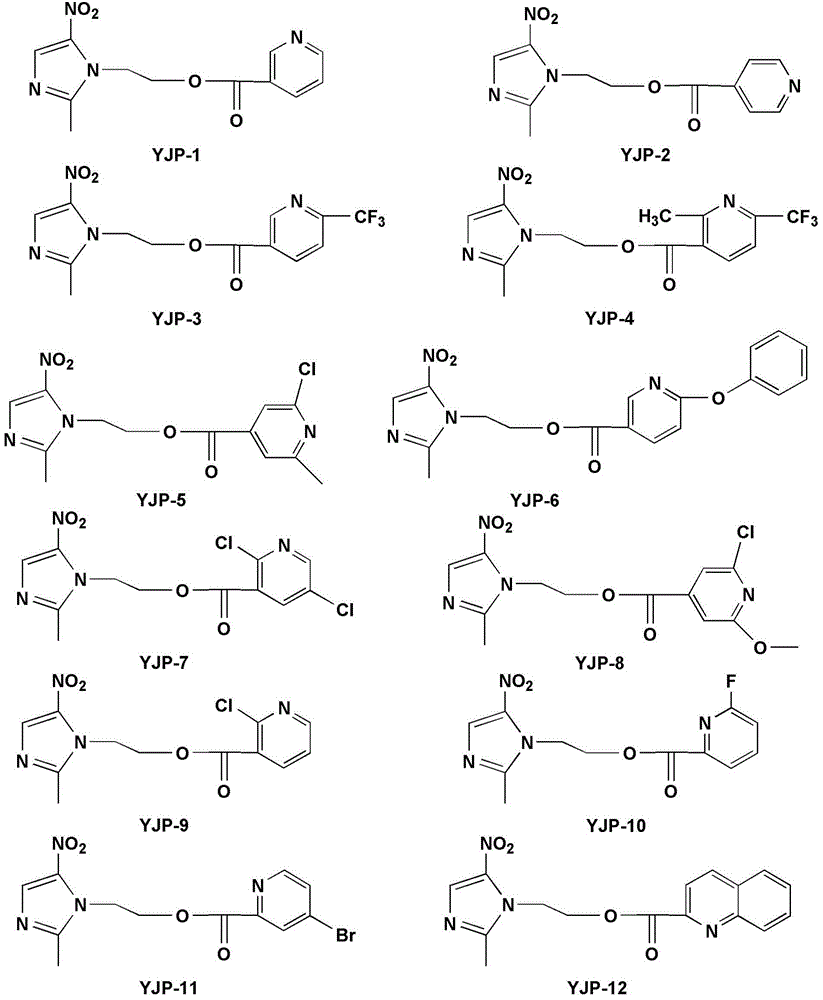
[0056] Only the reaction of nicotinic acid and metronidazole is taken as an example, and the preparation process of the rest of the class A metronidazole derivatives is similar to the reaction process of nicotinic acid and metronidazole, and will not be illustrated one by one in the examples.
[0057] Synthesis of metronidazole derivative YJP-1:
[0058]
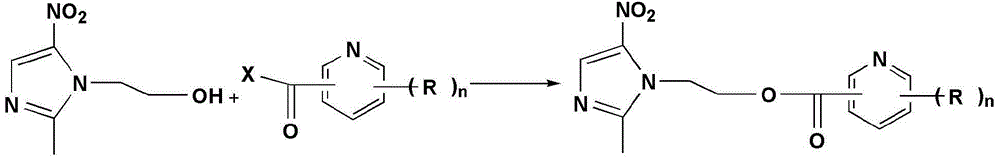
[0059] Add 0.123g (1mmol) of nicotinic acid and 0.206g (1mmol) of DCC into a 50mL round bottom flask, add 10mL of dry THF, stir and react in an ice bath for 30min, and dissolve 0.171g (1mmol) of metronidazole and 0.122g (1mmol) of ) 10 mL THF solution of DMAP was slowly added dropwise to the reaction system, stirred and reacted in an ice bath for 30 min, and then naturally rose to room temperature for reaction. After the completion of the TLC detection reaction, the reaction solution was concentrated in vacuo, and the residue was di...
Embodiment 2
[0063] The synthesis of the B class metronidazole derivative YJP-12 shown in embodiment 2 formula (I)
[0064]
[0065] Add 0.173g (1mmol) of 2-quinolinecarboxylic acid and 0.206g (1mmol) of DCC into a 50mL round-bottomed flask, add 10mL of dry THF, and stir in an ice bath for 30min to dissolve 0.171g (1mmol) of metronidazole and 0.122 A solution of g (1 mmol) DMAP in 10 mL THF was slowly added dropwise to the reaction system, stirred in an ice bath for 30 min, and then naturally rose to room temperature for reaction. After the completion of the TLC detection reaction, the reaction solution was concentrated in vacuo, and the residue was directly separated from the column. (石油醚) :V (乙酸乙酯) =5:1~2:1), the target compound YJP-12 was obtained.
[0066] The structures of the compounds prepared in Example 1-2 are all passed 1 H NMR, ESI-MS and other analytical methods were characterized. Their physical constants and spectral data are explained in tabular form:
[0067] The st...
Embodiment 3
[0073] Example 3 Anticancer Activity Test
[0074] MTT method was used to screen the activity against colorectal cancer cell line HCT-116, human lung cancer cell line A549 and breast cancer cell line MCF-7. The specific screening process is as follows:
[0075] (1) Spread the lung cancer cell line A549 in a 96-well plate, add 100 μL of culture medium, and wait for the cells to grow to 90%, add 1 μL of drugs into the wells, and detect 8 different concentrations of each drug (respectively, the initial concentration of the drug Concentration, 50μM, 5μM, 500nM, 50nM, 5nM, 500pM, 50pM), for each drug concentration, do 3 replicate wells in parallel, after culturing for 24h, add 20μL of the prepared 5mg / mL MTT solution to each well for 4 hours Afterwards, the medium was aspirated, and 150 μL of DMSO was added to each well, and the optical density (OD) value was measured at a wavelength of 595 nm. The negative control is DMSO. The inhibition rate was calculated according to the for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com