PD-1 antibody loaded T cell in-vitro culture method, cell preparation and application
A culture method and PD-1 technology, applied in the field of in vitro culture of T cells loaded with PD-1 antibody, can solve problems such as the toxic and side effects of PD-1 antibody drugs and the absence of PD-1 antibody drugs, so as to avoid loss of function and strengthen lethal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
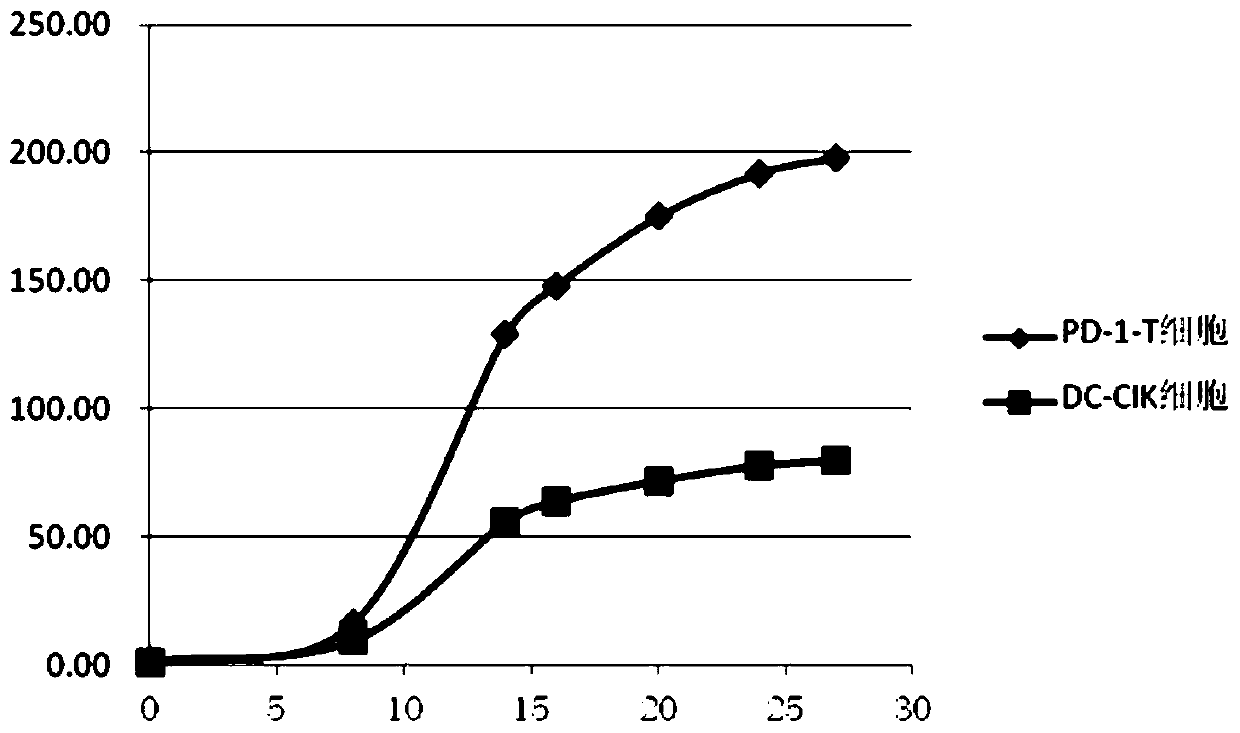
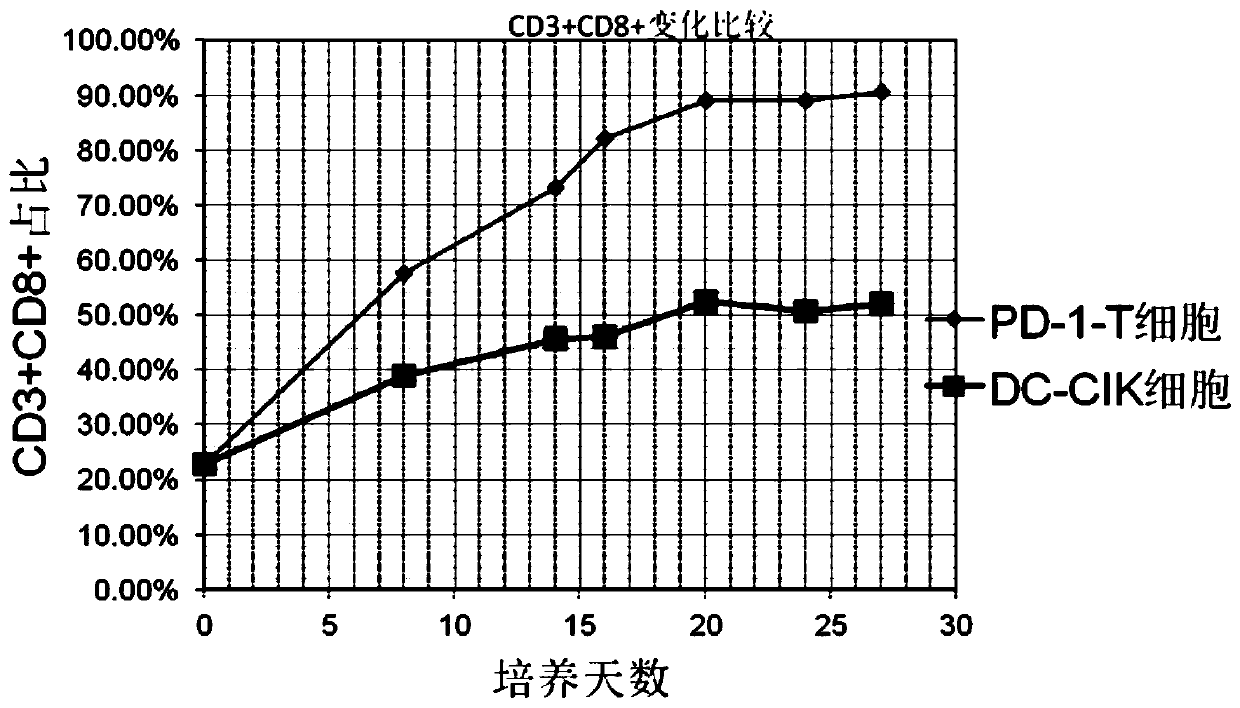
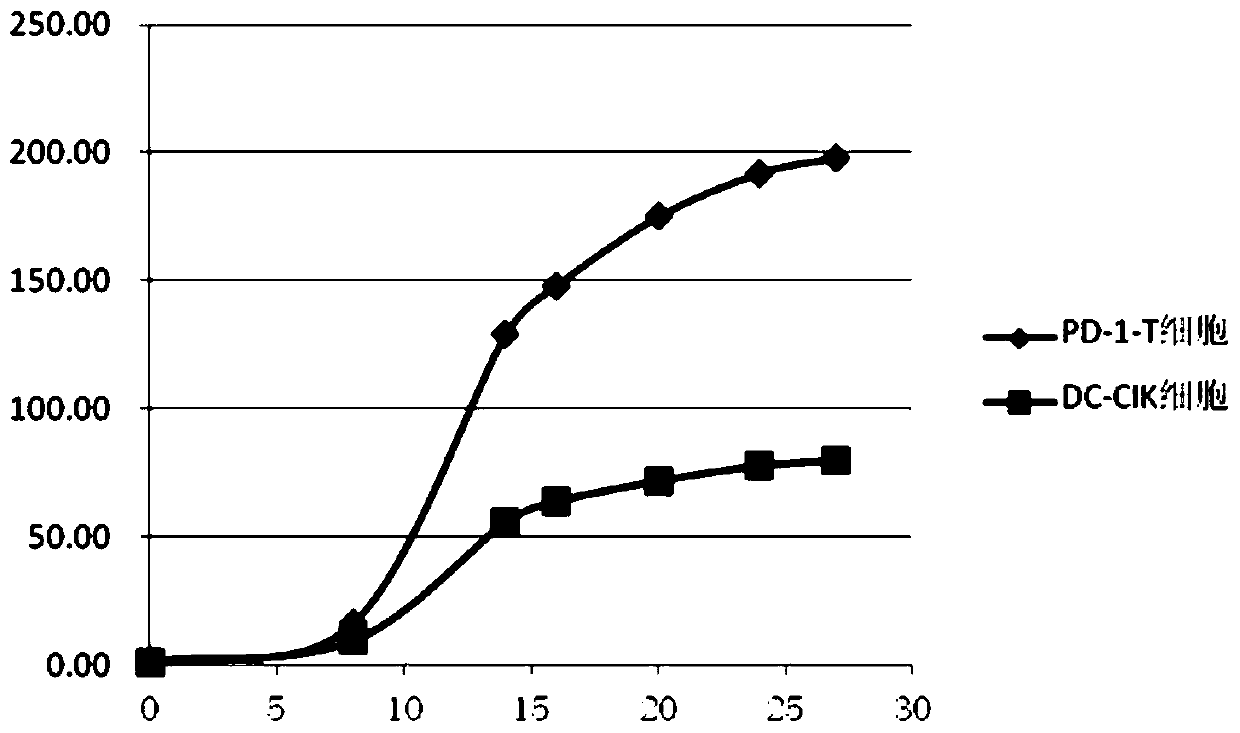
Image
Examples
Embodiment 1
[0056] The method of obtaining killer T cells is as follows.
[0057] Extract 150ml of peripheral blood, or collect cord blood, use lymphocyte separator and gradient centrifugation to separate mononuclear cells (mainly containing lymphocytes, as well as other white blood cells and a small amount of red blood cells).
[0058] First training
[0059] In the initial culture, the isolated mononuclear cells are mixed with anti-A medium, put into cell culture square flask, and placed at 37℃, 5% CO 2 Cultivate in a constant temperature and humidity carbon dioxide incubator.
[0060] The components of A anti-medium are as follows:
[0061] Complete medium, CD3 monoclonal antibody, interleukin-2, vitamin B6, interferon-γ, interleukin-1b, interleukin-4, L-glutamine, sodium propionate and plasma protein.
[0062] Among them, the final concentration of CD3 monoclonal antibody is 100ng / ml; preferably, in the A anti-medium, the final concentration of interleukin-2 is 1000u / ml, the final concentration ...
Embodiment 2
[0076] The effect of the cell preparation provided in Example 1 on patients with cervical cancer.
[0077] The results of the PET-CT examination of the cervical cancer patient on December 29, 2017 were:
[0078] 1. After cervical cancer, the uterus and bilateral appendages are absent; there are no signs of malignant tumors on the vaginal stump;
[0079] 2. The left supraclavicular fossa lymph node metastasis seen in the last imaging (2017-7-11), this imaging disappeared, the left supraclavicular fossa soft tissue disorder, slightly increased metabolism, it is considered as a post-treatment change;
[0080] 3. Going to several slightly enlarged lymph nodes in the middle and upper abdominal peritoneum, the metabolism is slightly increased, it is considered to be inflammation of the lymph nodes, and the metabolism is reduced compared with the previous imaging;
[0081] 4. A nodular hypermetabolic lesion in the upper segment of the left lateral lobe is considered as liver metastases;
[0082...
Embodiment 3
[0097] The efficacy of the cell preparation provided in Example 1 on leukemia patients was verified.
[0098] The leukemia patient was unwell at the end of 2017 and was diagnosed with monocytic leukemia (M5) in a local hospital. After receiving 5 chemotherapy sessions, the condition was relieved, but the blood cells remained low, accompanied by complications such as infection and physical discomfort. After seeking medical treatment from many sources, CIK immune cell therapy was accepted in July 2018, but with little effect. The applicant was contacted in August 2018 and received the first course of treatment on September 8, 2018 (one course of treatment and administration method: one A bottle of PD-1-T cell preparation contains 2.5-3 billion PD-1-T cells, and one bottle of PD-1-T cell preparation is injected every day for 7 consecutive days as a course of PD-1-T cell therapy. The results before and after treatment are as follows.
[0099] Inspection results before PD-1-T cell tran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com