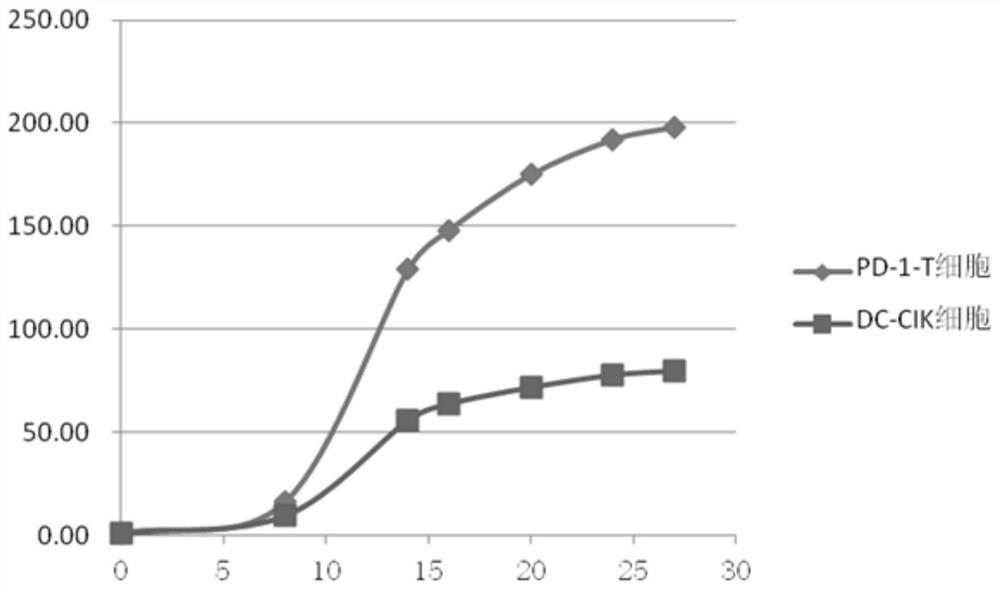
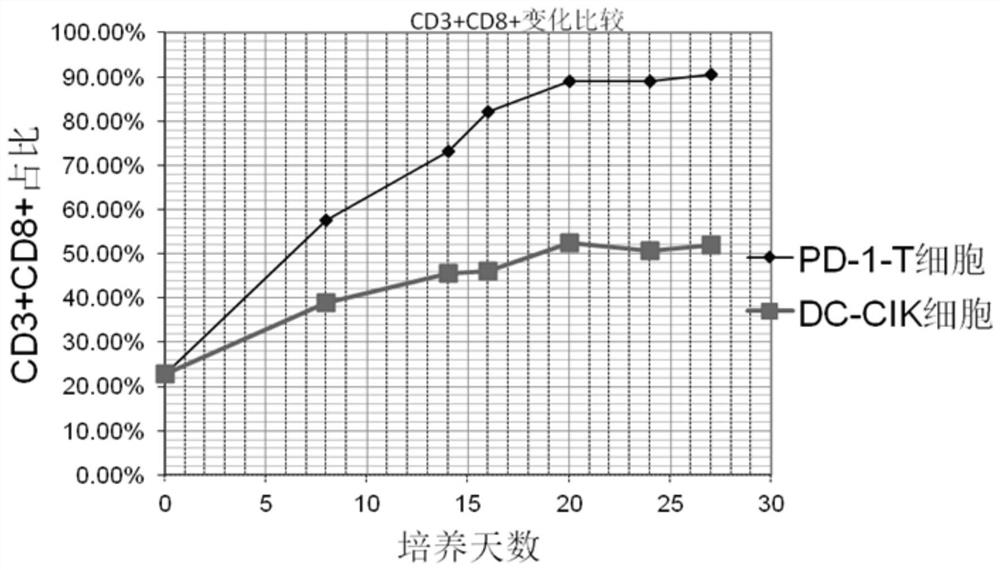
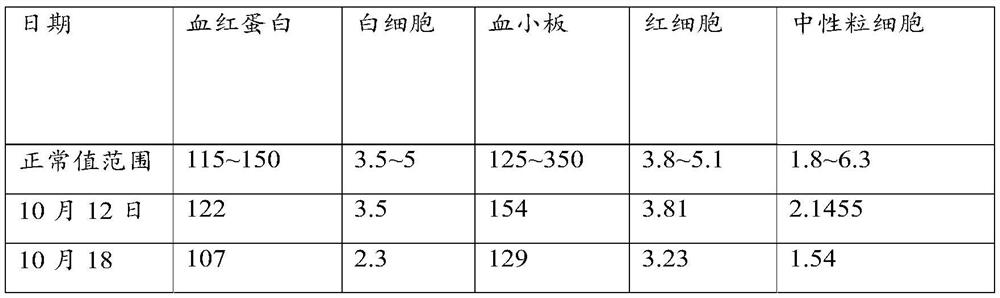
A method for in vitro culture of T cells loaded with PD-1 antibody, its cell preparation and its application
A culture method and PD-1 technology, applied in the field of in vitro culture of T cells loaded with PD-1 antibody, can solve problems such as the absence of PD-1 antibody drugs and the toxic and side effects of PD-1 antibody drugs, so as to enhance lethality and avoid Loss of function effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The method for obtaining killer T cells is as follows.
[0057] Take 150ml of peripheral blood, or collect umbilical cord blood, and use lymphocyte separation medium and gradient centrifugation to separate mononuclear cells (mainly including lymphocytes, as well as other white blood cells and a small amount of red blood cells).
[0058] first training
[0059] For the initial culture, the isolated mononuclear cells are mixed with anti-A medium, put into a cell culture square bottle, and placed at 37°C, 5% CO 2 cultured in a constant temperature and humidity carbon dioxide incubator.
[0060] The components of A anti-medium are as follows:
[0061] Complete medium, CD3 monoclonal antibody, interleukin-2, vitamin B6, interferon-γ, interleukin-1b, interleukin-4, L-glutamine, sodium propupuprate and plasma protein.
[0062] Wherein, the final concentration of CD3 monoclonal antibody is 100ng / ml; preferably, in the anti-A culture medium, the final concentration of interle...
Embodiment 2
[0076] The curative effect of the cell preparation provided in Example 1 for patients with cervical cancer.
[0077] The PET-CT examination results of the cervical cancer patient on December 29, 2017 were:
[0078] 1. After cervical cancer surgery, the uterus and bilateral appendages are absent; there is no sign of malignant tumor in the vaginal stump;
[0079] 2. The left supraclavicular fossa lymph node metastases seen in the last imaging (July 11, 2017) disappeared in this imaging, the left supraclavicular fossa soft tissue was disordered, and the metabolism was slightly increased, which was considered to be a change after treatment;
[0080] 3. Several slightly enlarged lymph nodes were removed from the retroperitoneum in the middle and upper abdomen, and the metabolism was slightly increased. It was considered lymph node inflammation, and the metabolism decreased compared with the previous imaging;
[0081] 4. A nodular hypermetabolism lesion in the upper part of the lef...
Embodiment 3
[0097] The curative effect of the cell preparation provided in Example 1 on leukemia patients was verified.
[0098] The leukemia patient was unwell at the end of 2017. He went to the local hospital for examination and was diagnosed with monocytic leukemia (M5). After receiving 5 times of chemotherapy, the condition was relieved, but the blood cells remained low, accompanied by complications such as susceptibility to infection and physical discomfort. After many medical consultations, he received CIK immune cell therapy in July 2018, but with little effect. In August 2018, he contacted the applicant and received the first course of treatment on September 8, 2018 (one course of treatment: one course of treatment) A bottle of PD-1-T cell preparation contains 2.5-3 billion PD-1-T cells, a bottle of PD-1-T cell preparation is injected every day, and 7 consecutive days is a course of treatment) PD-1-T cell therapy. The results before and after treatment are as follows.
[0099] On...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com