A strain of Rhodococcus rhodochrous and its application as an immune adjuvant in the preparation of vaccines
A technology of rhodococcus and vaccines, which is applied in the field of rhodococcus and its application as an immune adjuvant in the preparation of vaccines, and can solve the problems of unclear efficacy of inactivated vaccine immune adjuvants, low effect of slowly releasing antigens, human and animal Pathogenicity and other issues, to achieve the effect of good application prospects, short fermentation cycle, high immunostimulatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
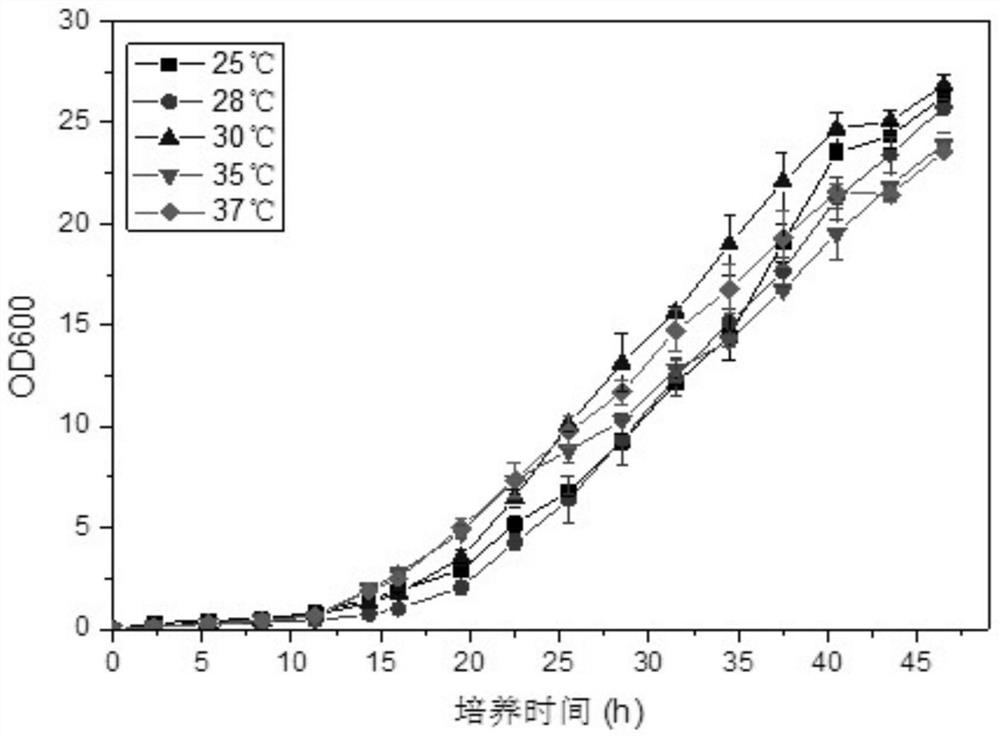
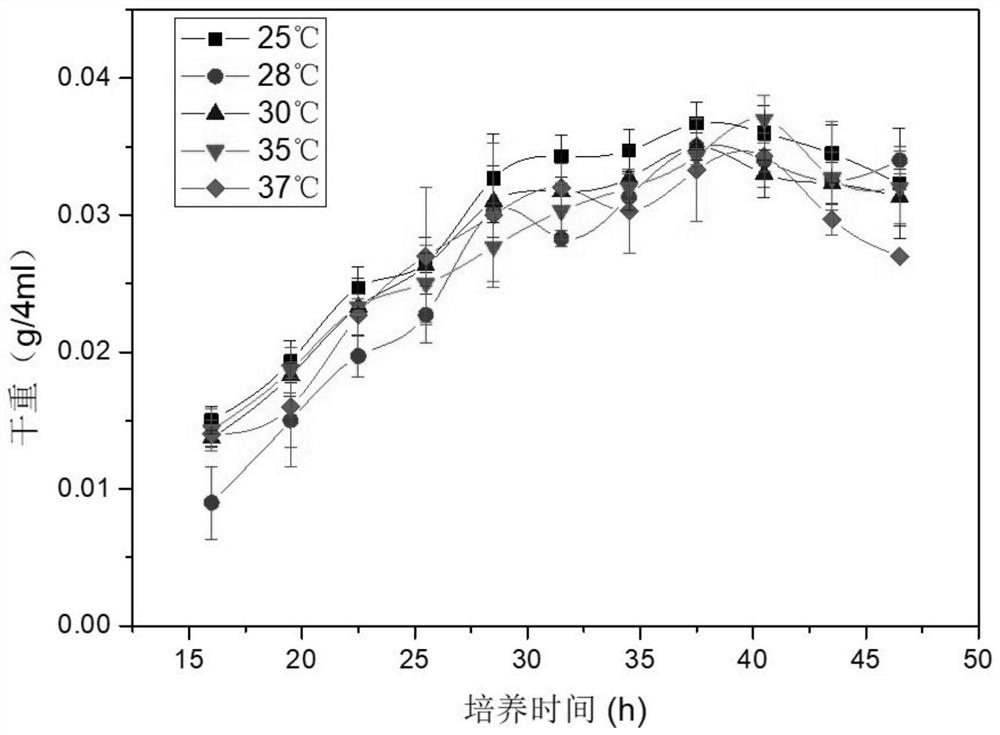
[0037] Example 1 (CGMCC NO.16640) Isolation and Identification of Rhodococcus ruber (Rhodococcus ruber) RDC-01
[0038] (1) Soil for testing: Soil samples were taken from the red soil in the suburbs of Guangzhou. The litter layer and roots on the soil surface were removed during sampling to avoid the influence of plants on the soil samples. The sampling depth is 5-10cm. The sampling time was late January 2017, passed through a 10-mesh sieve, and stored at 4°C.
[0039] (2) Strain screening: Take 10 g of fresh soil samples, put them into a conical flask filled with 90 mL of sterile water and glass beads, vibrate to make a bacterial suspension, and dilute it in a test tube. Choose three dilutions (10 -3 —10 -5 ) bacterial suspension was spread on LB medium, cultured at a constant temperature of 37°C, counted and observed the morphological characteristics of the colonies, and counted the types of autogenous bacteria in different soils. Select 6 well-growing strains, pick 5 si...
Embodiment 2
[0045] Embodiment 2 Neutral red method detects rhodococcus erythrococcus to mouse peritoneal macrophage Effects on Phagocytosis
[0046] Macrophages (abbreviated as ) are immune cells, It can activate and regulate lymphocytes or other immune cells to make them respond to pathogens, and can also secrete lymphokines, TNF, interferon, etc. to kill target cells. The neutral red method is to detect One of the commonly used detection methods for phagocytosis. The amount of neutral red phagocytized by macrophages can reflect its phagocytosis function, and the results are represented by A value.
[0047] (1) Experimental animals: 40 BALB / C mice, weighing 18-22 g, all male.
[0048] Medium: peptone 15g / L, yeast powder 15g / L, NaCl 10g / L, glucose 15g / L, NH 4 SO 4 2g / L, pH7.3.
[0049] 0.09% neutral red: Weigh 90 mg of neutral red biological dye, dissolve in 100 mL of normal saline, and sterilize under high pressure at 121°C.
[0050] Cell lysate: 0.1mol / L acetic acid: ethan...
Embodiment 3
[0058] The preparation of embodiment 3 H5 subtype avian influenza oil emulsion inactivated vaccine
[0059] (1) Adjuvant sample processing and quantification
[0060] Take the suspension of Rhodococcus erythrococcus RDC-01 made of sterile normal saline, shake it well, accurately measure 10mL into a glass petri dish, dry it in a blast drying oven at 80°C for 24h, and then weigh it every 1h , until the weight of the two weighings before and after is consistent, calculate the dry weight of the bacteria in the suspension, prepare the suspension with sterile saline to make an adjuvant solution of 100mg / mL (dry weight of the bacteria), and store it at 4°C for future use .
[0061] Take 10 mL of the above-mentioned Rhodococcus erythrococcus RDC-01 adjuvant solution, dissolve it in 10 mL of normal saline, prepare an adjuvant solution with a concentration of 50 mg / mL, and store it at 4°C for future use.
[0062] (2) Preparation of antigen (water phase):
[0063] ①Unadjuvanted contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com