Detection method for isothermal amplification of single or multiple target gene fragments
A gene fragment and target technology, which is applied in the field of amplifying the target gene fragment and detecting the amplification result, can solve the problems such as single amplification or multiple amplification detection is difficult to overcome, rapid detection cannot be realized, and promotion and use are restricted. Achieve strong multiple detection capabilities, fast detection speed, and easy results to read
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
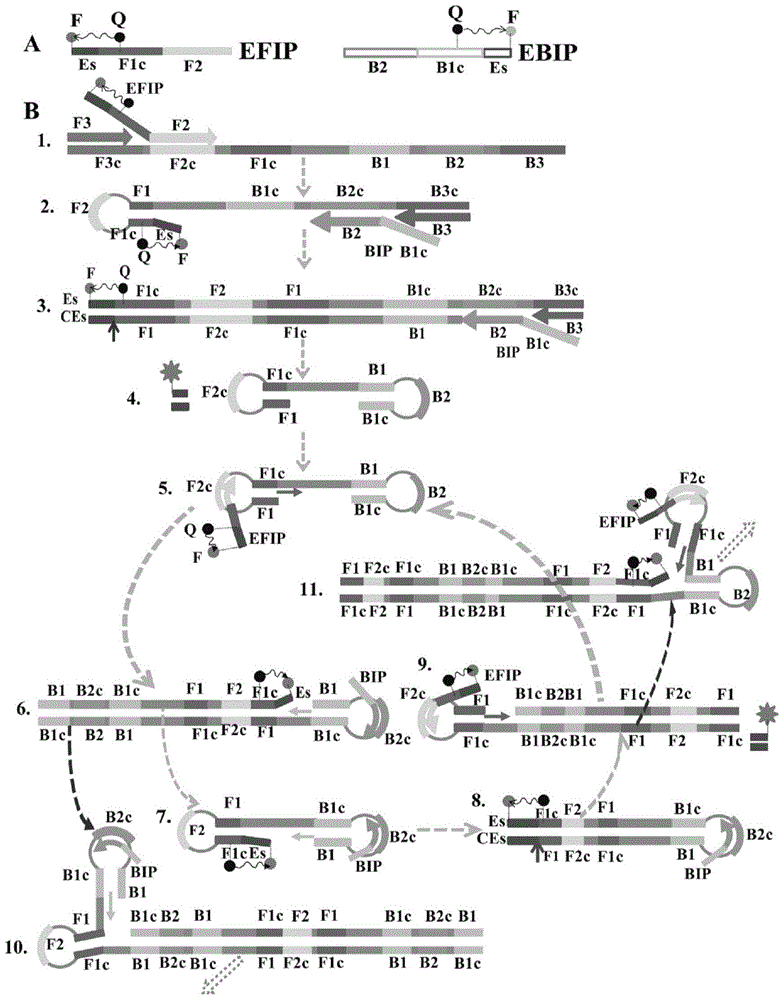
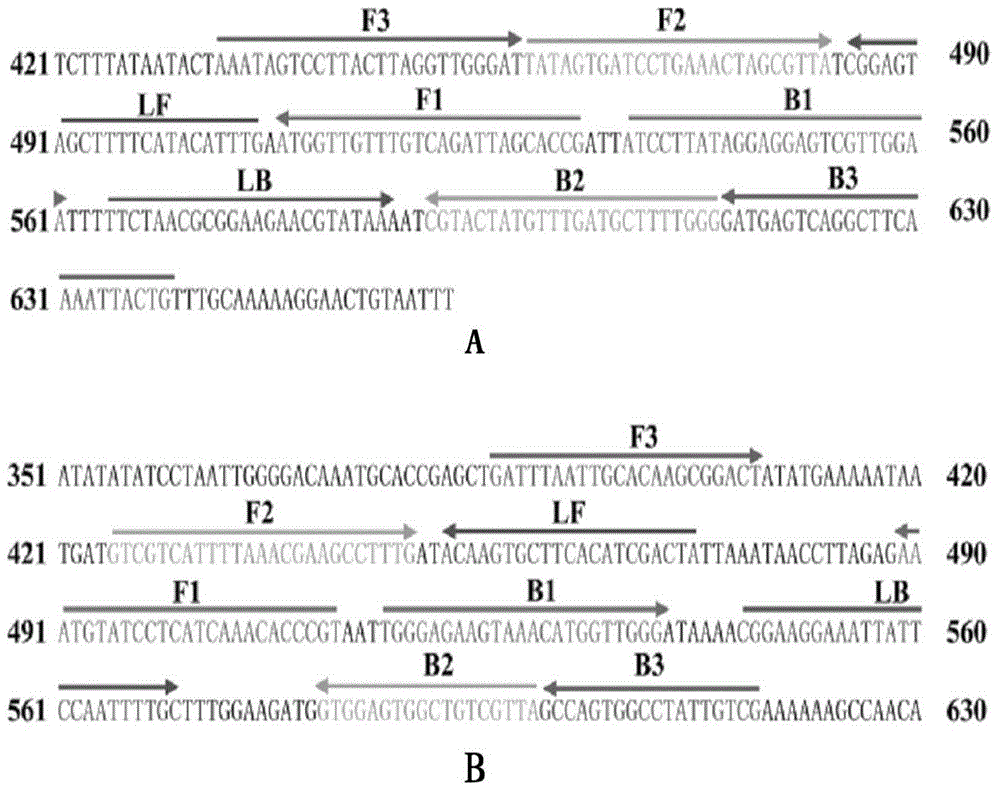
[0080] Example 1. Single amplification of MERT-LAMP:
[0081] (1) The MERT-LAMP single amplification (ie standard reaction) system is as follows: the concentration of primers EFIP and FIP are each 20 pmol, the concentration of primer BIP is 40 pmol, the concentration of primers F3 and B3 is 5 pmol, and the concentration of primers LF and BF is 20pmol, 10mM Betain, 6mM MgSO 4 , 1mM dNTP, 2.5μl of 10×Bst DNA polymerase buffer, 8U strand displacement DNA polymerase, 15U DNA restriction endonuclease, 1μl template, and add deionized water to 25μl. The whole reaction was carried out in a fluorescence detector (Rotor-Gene Q Real Time System, Qiagen), and the reaction was terminated at 63~65℃ for 1h by isothermal amplification and 5min at 80℃.
[0082] (2) Feasibility verification:
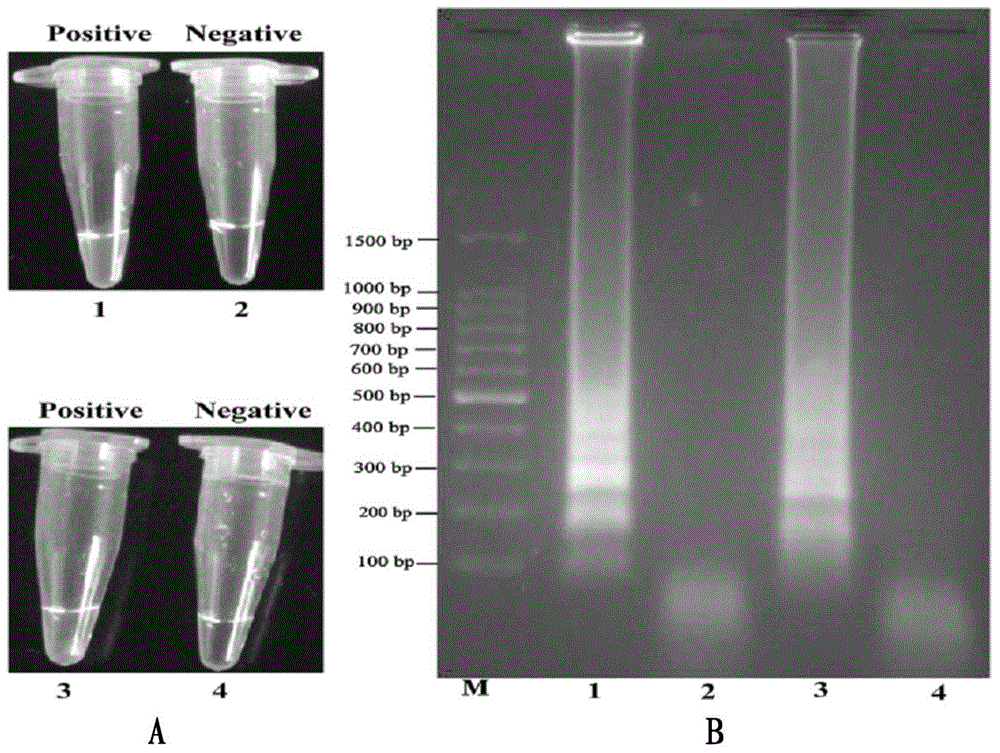
[0083] Under standard system conditions, the MERT-LAMP primer and corresponding DNA template designed according to lmo0733 and smcL specific genes are added, and the lmo0733-MERT-LAMP reaction is used to amplif...
Embodiment 2
[0093] Example 2. MERT-LAMP multiple amplification
[0094] (1) The MERT-LAMP multiple reaction system is as follows: the concentration of primers EFIP and FIP are each 20pmol, the concentration of primer BIP is 40pmol, the concentration of primers F3 and B3 are 10pmol, the concentration of primers LF and BF is 10pmol, 10mM Betain 6mM MgSO 4 , 1mM dNTP, 2.5μl of 10×Bst DNA polymerase buffer, 8U strand displacement DNA polymerase, 15U DNA restriction endonuclease, 1μl template, and add deionized water to 25μl. The whole reaction was carried out in a fluorescence detector (Rotor-Gene Q Real Time System, Qiagen), and the isothermal amplification was carried out at 64°C for 1 h and 80°C for 5 min to terminate the reaction.
[0095] (2) Verify the multiple detection capability and sensitivity of MERT-LAMP
[0096] In order to realize the multiple amplification detection of MERT-LAMP technology, the present invention optimizes the standard system to adapt to multiple detection, and establ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com