Tetrahydroisoquinoline compound intermediate, and preparation method and application thereof
A technology for tetrahydroisoquinoline and compounds, which is applied in the field of tetrahydroisoquinoline compound intermediates, can solve the problems of high price, low total yield, numerous and complicated reaction steps, etc., and achieves low-cost raw materials, simple preparation methods, The effect of high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
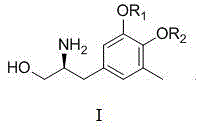
[0063] The preparation method of the tetrahydroisoquinoline compound of the present invention comprises the following steps: taking the halogenated aromatic compound as the starting material, undergoing nucleophilic substitution, addition, hydroxyl elimination and protecting group removal reactions to obtain a chiral The tetrahydroisoquinoline compound of amine, preparation process is as follows.
[0064]
[0065] The preparation method of the tetrahydroisoquinoline compound specifically includes the following steps.
[0066] A. Preparation of D-cyclic aldehydes.
[0067] The preparation of the D-cyclic aldehyde is as follows.
[0068]
[0069] (a) Preparation of N-benzyloxycarbonyl-D-serine methyl ester (N-Cbz-D-serine methyl ester).
[0070] The raw material compound 5 was mixed with methanol (CH 3 OH), thionyl chloride (SOCl 2 ) reaction to generate D-serine methyl ester hydrochloride.
[0071] In the present embodiment, the raw material compound 5 is D-serine, ...
Embodiment 1
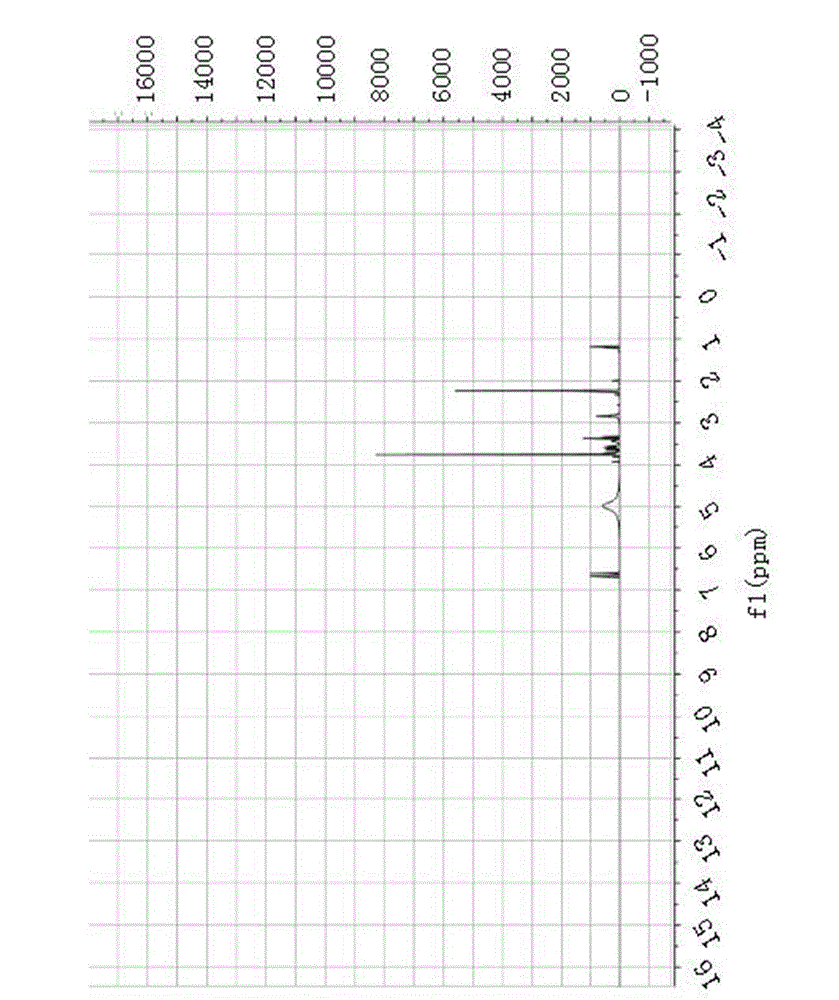
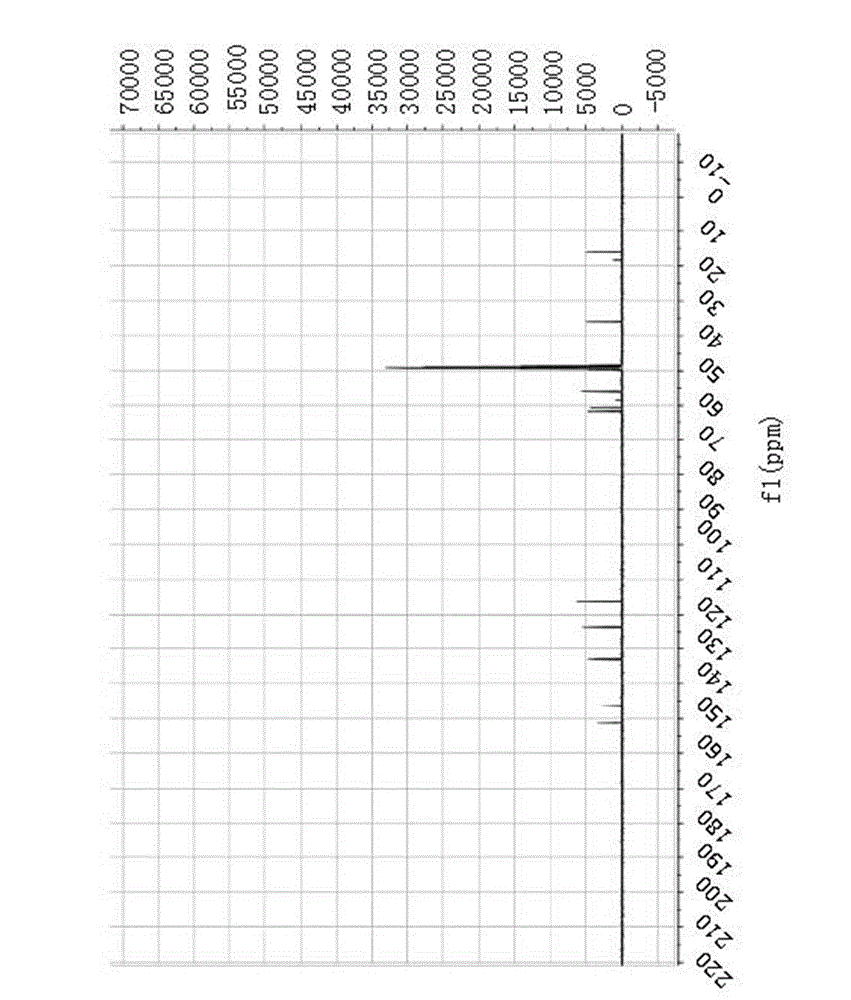
[0102] Example 1 Preparation of (S)-5-(2-amino-3-hydroxypropyl)-2-methoxy-3-methylphenol
[0103] The following embodiments will help to further understand the present invention, but are not limited to the content of the present invention. The preparation process of the tetrahydroisoquinoline compound (S)-5-(2-amino-3-hydroxypropyl)-2-methoxy-3-methylphenol of the present invention is as follows.
[0104]
[0105] A. To prepare N-benzyloxycarbonyl-D-serinaldehyde, the preparation process is as follows.
[0106]
[0107] (a) Preparation of N-Cbz-D-serine methyl ester.
[0108] 200 mL CH 3 OH was placed in a 250 mL three-neck flask, cooled to 0 °C, and 22.0 mL SOCl 2 Add dropwise into methanol, react for 30 min, add 21.1 g of D-serine into the reaction system, slowly rise to room temperature and react overnight. The reaction solution was spin-dried to obtain 30 g of D-serine methyl ester hydrochloride with a yield of 98%.
[0109] Take 15.5 g of D-serine methyl este...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com