A kind of gel electrochromic material and preparation method thereof
An electrochromic material and gel technology, applied in the chemical field, can solve problems such as density changes that cannot be solved, and achieve the effects of solving the problem of long-term discoloration and migration, improving service life, and stabilizing relative positions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 electrochromic material is carried out in five steps:
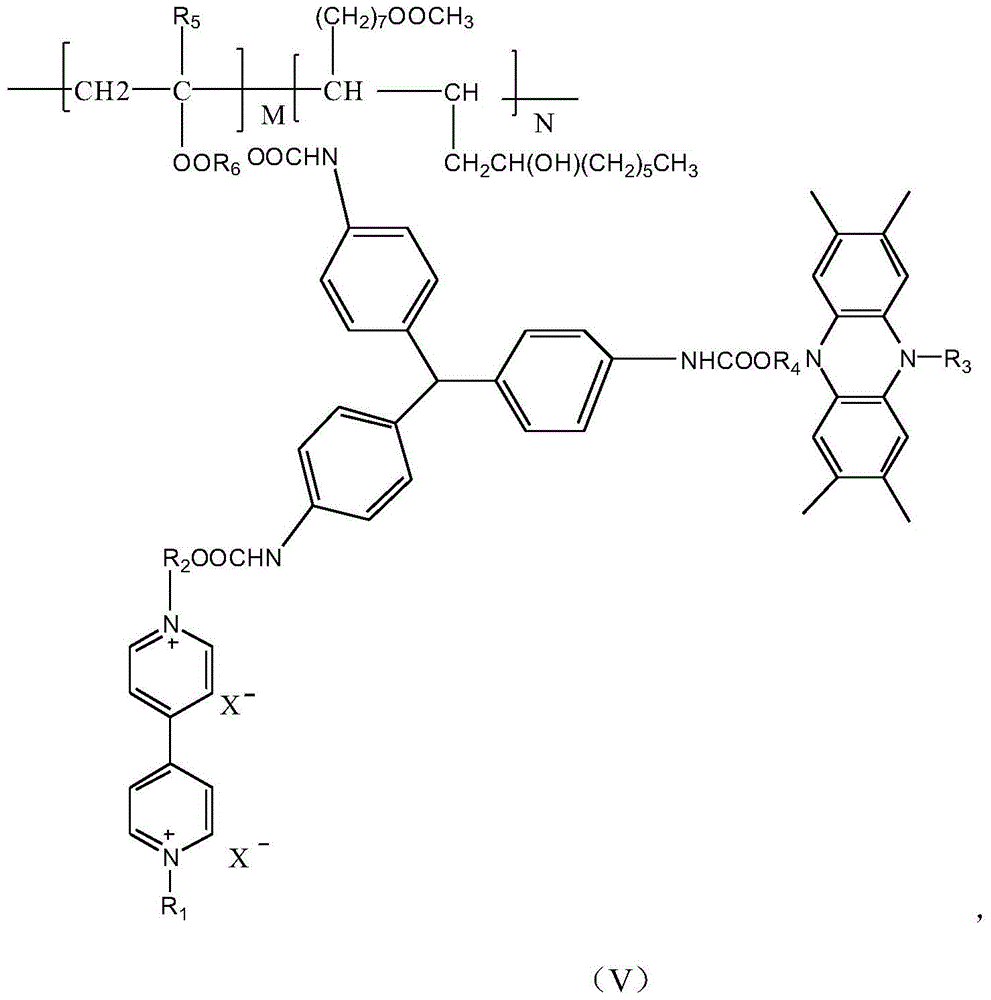
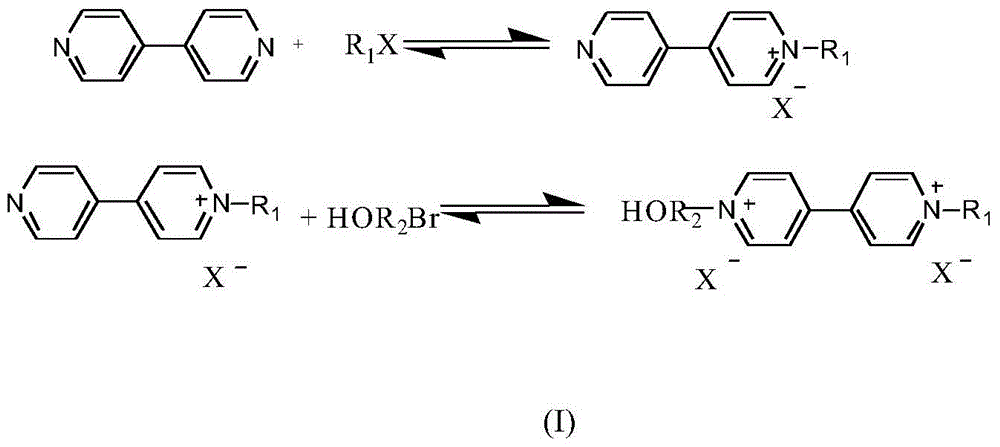
[0030] (1) Dissolve 0.1 mol of 4,4'-bipyridine and 0.1 mol of bromoethane in 250 ml of acetonitrile solution, heat and reflux under stirring for 24 hours, put it in the refrigerator to cool, and filter after the crystals are precipitated to obtain About 0.075mol of 4-ethyl 4,4'-bipyridine derivatives; reacting 0.05mol of the above-mentioned monosubstituted 4,4'-bipyridine derivatives with 0.05mol of hexabromo-n-hexanol to obtain double bond-containing 4,4'-bipyridine disubstituted derivatives (I);
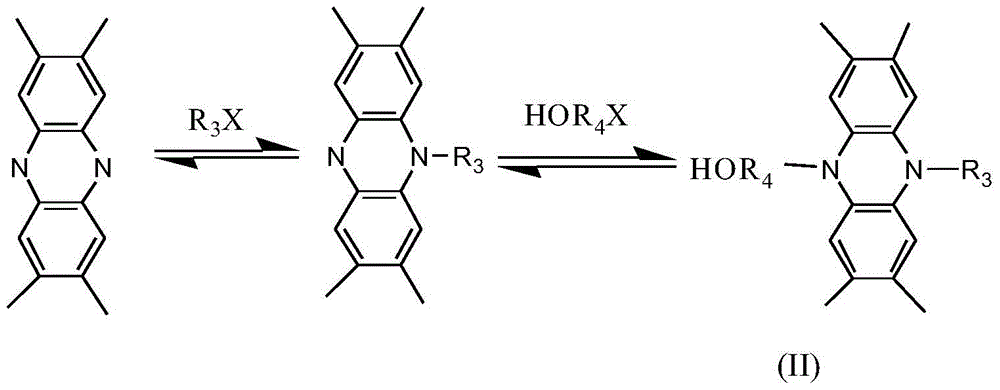
[0031] (2) 0.1mol of phenazine derivatives Dissolve 0.1 mol of bromoethane in 250 ml of acetonitrile solution, heat and reflux under stirring for 8 hours, put it in the refrigerator to cool, and filter after the crystals are precipitated to obtain about 0.06 mol of monosubstituted phenazine derivatives; 0.05 mol of The above-mentioned monosubstituted phenazine derivatives are reacted w...
Embodiment 2
[0035] The preparation of embodiment 2 electrochromic material is carried out in five steps:
[0036] (1) Dissolve 0.1mol of 4,4'-bipyridine and 0.1mol of bromobutane in 250ml of acetonitrile solution, heat and reflux under stirring for 24 hours, put it into the refrigerator to cool, and filter after the crystals are precipitated to obtain about 0.075mol of 4-butyl 4,4'-bipyridine derivatives; the above-mentioned monosubstituted 4,4'-bipyridine derivatives of 0.05mol are reacted with 0.05mol of hexabromo-n-hexanol to obtain 4 , 4'-bipyridine disubstituted derivatives (I);
[0037] (2) 0.1mol of phenazine derivatives Dissolve 0.1 mol of bromoethane in 250 ml of acetonitrile solution, heat and reflux under stirring for 8 hours, put it in the refrigerator to cool, and filter after the crystals are precipitated to obtain about 0.06 mol of monosubstituted phenazine derivatives; 0.05 mol of The above-mentioned monosubstituted phenazine derivatives are reacted with 0.05mol of brom...
Embodiment 3
[0041] Embodiment 3 The preparation of the electrochromic material is carried out in five steps:
[0042] (1) Dissolve 0.1mol of 4,4'-bipyridine and 0.1mol of bromoheptane in 250ml of acetonitrile solution, heat and reflux under stirring for 24 hours, put it in the refrigerator to cool, and filter after the crystals are precipitated to obtain about 0.075mol of 4-heptyl 4,4'-bipyridine derivatives; 0.05mol of the above-mentioned monosubstituted 4,4'-bipyridine derivatives reacted with 0.05mol of hexabromo-n-hexanol to obtain 4 containing double bonds , 4'-bipyridine disubstituted derivatives (I);
[0043] (2) 0.1mol of phenazine derivatives Dissolve 0.1 mol of bromoethane in 250 ml of acetonitrile solution, heat and reflux under stirring for 8 hours, put it in the refrigerator to cool, and filter after the crystals are precipitated to obtain about 0.06 mol of monosubstituted phenazine derivatives; 0.05 mol of The above-mentioned monosubstituted phenazine derivatives are reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com