Nitric acid two-step bleaching technology and device
A process, the technology of nitric acid, applied in the field of nitric acid production and preparation, can solve the problems of consuming a large amount of electric energy or heat energy, taking a long time, and not being able to solve dilute nitric acid, etc., and achieve the effect of rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
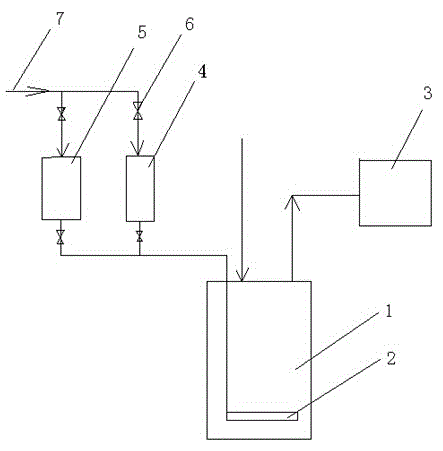
[0032] Such as figure 1 As shown, a device for the bleaching process in the preparation process of nitric acid includes an acid distribution tank 1, the top outlet of the acid distribution tank 1 is connected to a negative pressure pump 3, and the inlet end of the acid distribution tank 1 is connected to a hydrogen peroxide storage tank 4 respectively. 1. The outlet end of the urea solution storage tank 5 is connected, the top inlets of the hydrogen peroxide storage tank 4 and the urea solution storage tank 5 are all connected with the compressed air pipe 7, and the inside of the acid distribution tank 1 is horizontally provided with a gas distributor 2. Regulating valves 6 are provided at the inlet and outlet ends of the hydrogen peroxide storage tank 4 and the urea solution storage tank 5 .
[0033] That is, first open the regulating valve 6 on the inlet and outlet ends of the hydrogen peroxide storage tank 4, the hydrogen peroxide in the hydrogen peroxide storage tank 6 is ...
Embodiment 2
[0035] A kind of bleaching process in the preparation process of nitric acid, comprises the following steps:
[0036] (1) Pump the acid tank for preparing nitric acid to a slight negative pressure of -10Pa;
[0037] (2) With compressed air as the power, hydrogen peroxide is introduced into the nitric acid solution in the acid distribution tank, and the hydrogen peroxide enters the nitric acid solution evenly through the gas distributor and stirred evenly, so that the hydrogen peroxide and the NO in the acid distribution tank 2 The molar ratio is 1.5:2;
[0038] (3) Recover excess NO at the outlet of the acid distribution tank 2 ;
[0039] (4) Evenly add urea solution with a concentration of 32.5% into the acid mixing tank, the mass of urea in the urea solution accounts for 1 / 10,000 of the total mass of nitric acid in the acid mixing tank.
Embodiment 3
[0041] A kind of bleaching process in the preparation process of nitric acid, comprises the following steps:
[0042] (1) Pump the acid tank for preparing nitric acid to a slight negative pressure of -5Pa;
[0043](2) With compressed air as the power, hydrogen peroxide is introduced into the nitric acid solution in the acid distribution tank, and the hydrogen peroxide enters the nitric acid solution evenly through the gas distributor and stirred evenly, so that the hydrogen peroxide and the NO in the acid distribution tank 2 The molar ratio is 1.2:2;
[0044] (3) Recover excess NO at the outlet of the acid distribution tank 2 ;
[0045] (4) Evenly add urea solution with a concentration of 32.5% into the acid mixing tank, the mass of urea in the urea solution accounts for 1 / 10,000 of the total mass of nitric acid in the acid mixing tank.
[0046] In the present invention, hydrogen peroxide and urea are used to uniformly enter the solution in the acid distribution tank throug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com