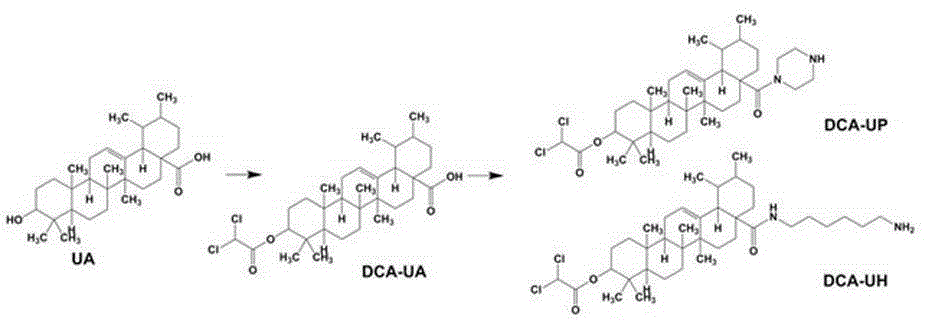
Ursolic acid-glycolysis inhibitor DCA conjugate and application thereof
A technology of ursolic acid and its application, which is applied in the field of ursolic acid derivatives and its application, can solve the problems of low bioavailability, and achieve the effect of excellent proliferation inhibition and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of DCA-UA
[0026] At room temperature, dissolve 0.5 g UA in 20 mL 1v / 1v pyridine:dichloromethane mixed solution, add 0.1eq DMAP, and slowly add 108 μL dichloroacetyl chloride dropwise to the aforementioned solution under magnetic stirring conditions, and the dropwise addition is completed Then continue to stir for 8-10h. After the reaction was complete, dichloromethane was distilled off under reduced pressure. Add 100 mL of water to the reaction flask to precipitate the product, filter with suction, wash the filter cake with 500 mL of water until neutral, dry in vacuum, and obtain DCA-UA by column chromatography.
[0027] Properties: White powder; Yield: 70.51%
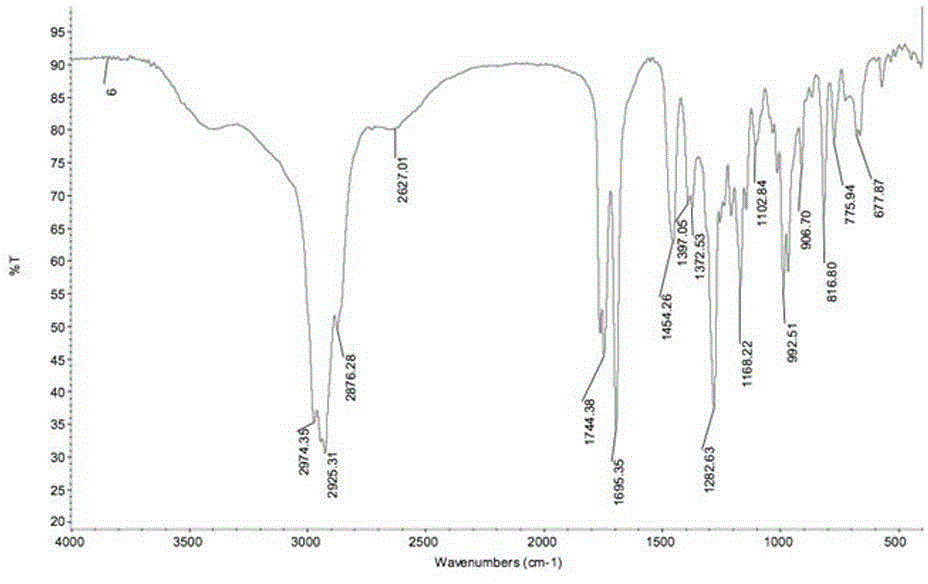
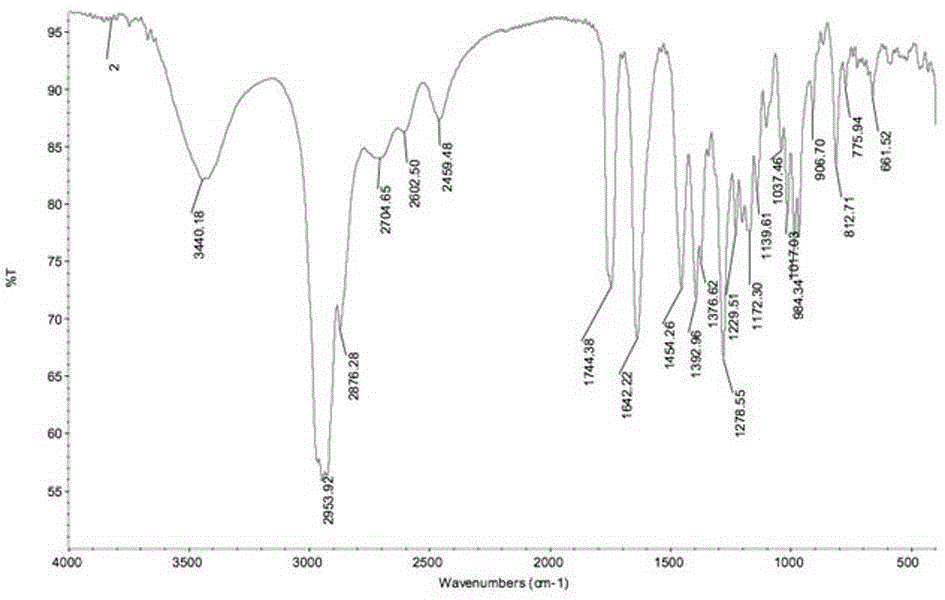
[0028] IR data: see 2
[0029] HRMS: Theoretical value: 565.2857 m / z Actual value: 565.2860 m / z
[0030] 1 H NMR (400 MHz, CDCl 3 ) δ 5.97 (d, J = 2.9 Hz, 1H), 5.26 (d, J = 2.2 Hz, 1H), 4.67 (dd, J = 20.3, 18.0 Hz, 1H), 2.40 – 2.15 (m, 1H), 2.09 – 1.84 (m, 4H), 1.74 (s, 6H), ...
Embodiment 2
[0031] Embodiment 2: the synthesis of DCA-UP
[0032] At room temperature, 0.5 g of DCA-UA was dissolved in 20 mL of dichloromethane. Under the condition of magnetic stirring, 0.6 mL of oxalyl chloride was slowly added dropwise to the above solution, and the stirring was continued for 8-10 h after the dropwise addition was completed. After the reaction was complete, the gas and solvent were distilled off under reduced pressure. Under magnetic stirring, the aforementioned product was dissolved in 20 mL of dichloromethane, and slowly added dropwise to a solution of 0.4 g of piperazine and 100 μL of triethylamine in 20 mL of dichloromethane. After the dropwise addition was completed, stirring was continued for 12h. After the reaction was complete, 20 mL of dichloromethane was added to the reaction flask, and the reaction system was extracted 2-4 times with 50 mL of 1N HCl solution until the pH was 3-4. The organic layer was collected by liquid separation, dried over anhydrous so...
Embodiment 3
[0037] Embodiment 3: the synthesis of DCA-UH
[0038] At room temperature, 0.5 g of DCA-UA was dissolved in 20 mL of dichloromethane. Under the condition of magnetic stirring, 0.6 mL of oxalyl chloride was slowly added dropwise to the above solution, and the stirring was continued for 8-10 h after the dropwise addition was completed. After the reaction was complete, the gas and solvent were distilled off under reduced pressure. Under magnetic stirring, the aforementioned product was dissolved in 20 mL of dichloromethane, and slowly added dropwise to a solution of 0.5 g of hexamethylenediamine and 100 μL of triethylamine in 20 mL of dichloromethane. After the dropwise addition was completed, stirring was continued for 12h. After the reaction was complete, 20 mL of dichloromethane was added to the reaction flask, and the reaction system was extracted 2-4 times with 50 mL of 1N HCl solution until the pH was 3-4. The organic layer was collected by liquid separation, dried over an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com