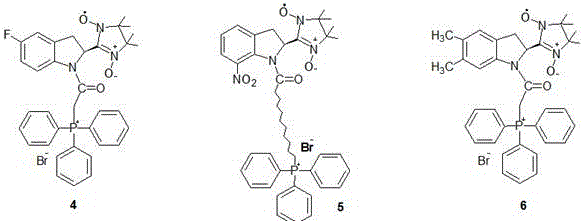
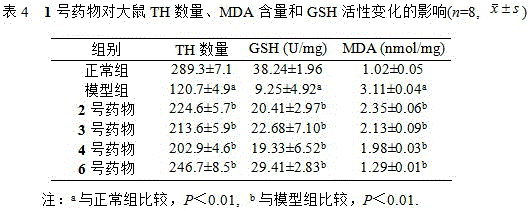
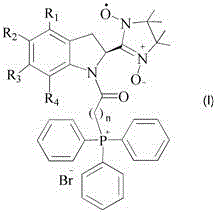
Mitochondrial function protective agent and its preparation method and application
A route and reaction technology, applied in the field of medicine, can solve the problems of limited oxidative damage resistance to neurodegenerative diseases, insufficient efficacy, poor selectivity of antioxidants, etc., to protect neuron cells, clarify the protective effect, and efficiently remove harmful free radicals Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthetic method of compound 1
[0024] In 4.41 g (30 mmol) 1H-indole-2-carbaldehyde and added to 100 mL CH 2 Cl 2 4.4 mL of triethylamine was added to the reaction mixture, stirred magnetically in an ice bath, and then 30 mL of CH containing 2.01 g (30 mmol) of bromoacetyl bromide was slowly added dropwise to the reaction solution. 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was removed to obtain a white solid product, which was purified by column chromatography to obtain 7.16 g of the product, with a yield of 89%. MS( m / z ): 266.01[M] + ; 1 H NMR (CDCl 3 ): d 9.25(s, 1H), 7.23(d, 1H), 7.20(d, 1H), 7.14(s, 1H), 7.04(s, 1H), 4.90(m, 1H), 4.25(s, 1H ), 3.11(d, 2H); Anal. Calcd for C 11 h 10 BrNO 2 : C,49.28; H, 3.76; ...
Embodiment 2
[0027] Embodiment 2: the synthetic method of compound 2
[0028] In 6.21 g (30 mmol) of 5-methoxy-6-methoxy-1H-indole-2-carbaldehyde and added to 100 mL of CH 2 Cl 2 Add 4.4 mL of triethylamine, stir magnetically under ice bath, then slowly add 30 mL of CH containing 2.01 g (30 mmol) bromopropionyl bromide dropwise to the reaction solution 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, and the organic phase was washed with anhydrous MgSO 4After drying, the solvent was removed to obtain a white solid product, which was purified by column chromatography to obtain 7.98 g of the product, with a yield of 78%. MS( m / z ): 341.62 [M] + ; 1 H NMR (CDCl 3 ): d 9.59 (d, 1H), 6.84(s, 1H), 6.73(s, 1H), 4.87(m, 1H), 3.81(s, 6H), 3.71(m, 2H), 3.15(m, 2H ), 2.74(m, 2H); Anal. Calcd for C 14 h 16 BrNO 2 : C,49.14; H, 4.71; N,4.09, Found: C,49...
Embodiment 3
[0031] Embodiment 3: the synthetic method of compound 5
[0032] In 5.76 g (30 mmol) of 7-nitro-1H-indole-2-carbaldehyde and added to 100 mL CH 2 Cl 2 Add 4.4 mL of triethylamine, stir magnetically under ice bath, then slowly add 30 mL of CH containing 2.01 g (30 mmol) bromoundecanoyl bromide to the reaction liquid 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was removed to obtain a white solid product, which was purified by column chromatography to obtain 8.14 g of the product, with a yield of 62%. MS( m / z ): 438.23[M] + ; 1 H NMR (CDCl 3 ): d 9.72(d, 1H), 8.17(d, 1H), 7.53(d,1H), 7.39(d, 1H), 4.83(m, 1H), 3.32(m, 2H) 3.15(d, 2H) , 2.24~1.23(m, 18H); Anal. Calcd for C 20 h 27 BrN 2 o 4 : C,54.68; H, 6.19; N,6.38, Found: C,54.62; H,6.23; N,6.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com