Antibacterial peptide His6K and application thereof
An anti-microbial and fusion peptide technology, applied to the antibacterial peptide His6K and its application fields, can solve the problems of low content of natural antibacterial peptides, difficulty in extraction, and high cost of chemically synthesized antibacterial peptides, and achieve the effect of good antibacterial activity and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, artificial synthesis of antibacterial peptide and antibacterial activity assay
[0050] 1. Synthesis of antimicrobial peptides
[0051] The amino acid sequence of the antimicrobial peptide in the prior art is shown in sequence 1 of the sequence listing. The antimicrobial peptide shown in Sequence 1 of the Sequence Listing was artificially synthesized and named as the antimicrobial peptide Histonin.
[0052] The amino acid sequence of the antimicrobial peptide provided by the present invention is shown in sequence 2 of the sequence listing, and its coding gene is shown in sequence 3 of the sequence listing. The antimicrobial peptide shown in Sequence 2 of the Sequence Listing was artificially synthesized and named as the antimicrobial peptide His6K.
[0053] Second, the determination of the minimum inhibitory concentration MIC of antimicrobial peptides
[0054] Detect the minimum inhibitory concentration MIC of antimicrobial peptides (antimicrobial peptide...
Embodiment 2
[0061] Embodiment 2, the preparation of antimicrobial peptide His6K and antibacterial activity assay
[0062] 1. Construction of recombinant plasmids
[0063] 1. Synthesize the double-stranded DNA molecule shown in the sequence 4 of the sequence listing from the 244-746th nucleotide at the 5' end.
[0064] 2. Double-digest the double-stranded DNA molecule obtained in step 1 with restriction endonucleases XhoI and NotI, and recover the digested product.
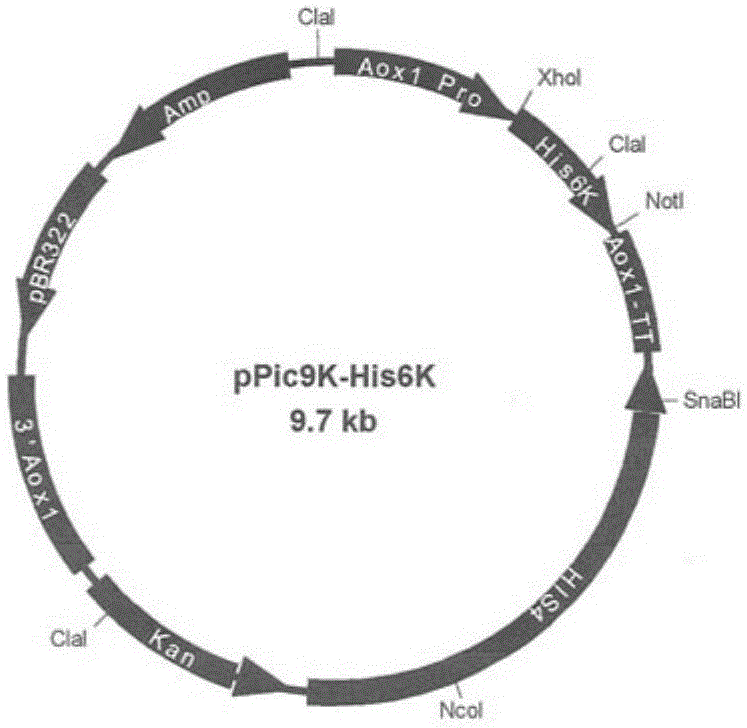
[0065] 3. Digest the yeast expression vector pPic9K with restriction endonucleases XhoI and NotI, and recover the vector skeleton of about 9.3 kb.
[0066] 4. Ligate the digested product obtained in step 2 with the vector backbone obtained in step 3 to obtain the recombinant plasmid pPic9K-His6K ( figure 1 ). For the results of enzyme digestion and identification of the recombinant plasmid pPic9K-His6K, see figure 2 ( figure 2 Among them, M is DNA molecular weight marker; Lane 1 is the result of restriction endonuclease...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com