Antibacterial peptide MAMP-03 and application thereof
A technology of MAMP-03 and antimicrobial peptides, which is applied in antibacterial drugs, peptides, preparations for toiletry, etc., can solve the problems of limited effect, large cationic antibacterial peptides, and toxic effects, and achieve good application prospects and good antibacterial active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation and identification of hydrolyzed milk protein peptides.
[0014] (1) Sample preparation
[0015] Take 1g of cow casein and dissolve it in 10ml of water, add 20mg of trypsin and react at 37°C for 4 hours. After the enzymolysis process, centrifuge at 14000×g and 4°C for 20min, and the supernatant is the hydrolyzed milk protein peptide sample.
[0016] (2) LC-MS / MS analysis
[0017] The hydrolyzed milk protein peptide solution sample was desalted with a C18 column (Waters Oasis HLB SPE column, 1cc / 30mg, 30um) according to the following steps: the C18 column was activated with 1.5mL methanol and added 1.5mL 0.1% (V / V) TFA (trifluoro Acetic acid)-H2O solution balance, the sample was redissolved with 1ml 0.1% (V / V) TFA-H2O solution and added to the C18 column, and 1.5mL 80% (V / V) ACN (acetonitrile) / 0.1% (V / V) TFA-H2O solution was used for elution, and the eluate was collected, subpackaged, freeze-dried and stored at -80°C. The desalted sample was dissolved in ...
Embodiment 2
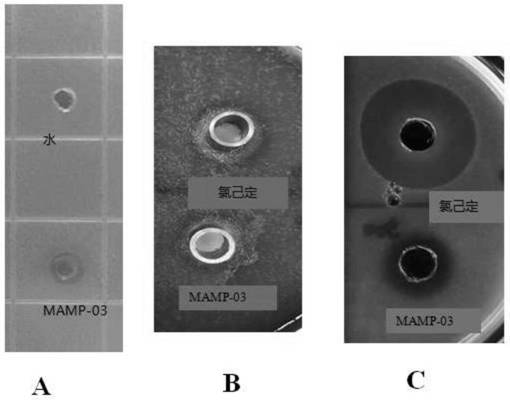
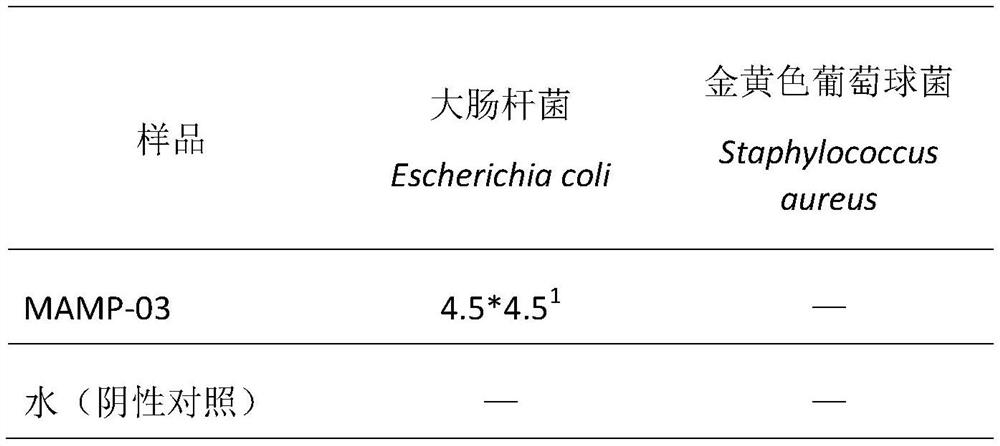
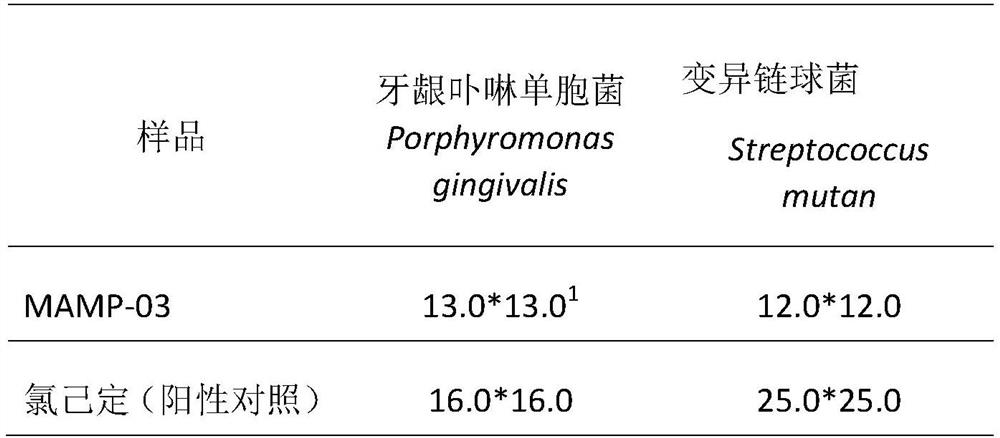
[0032] Antibacterial activity of peptides.
[0033] The peptide MAMP-03 was chemically synthesized in Shanghai Jiepeptide Technology Co., Ltd. with a purity of 97.6%. Escherichia coli and Porphyromonas gingivalis in Gram-negative bacteria, Staphylococcus aureus and Streptococcus mutans in Gram-positive bacteria were used as target strains to investigate the antibacterial activity of polypeptide MAMP-03.
[0034] (1) Strains and recovery
[0035] Escherichia coli (Escherichia coli K12), Staphylococcus aureus (Staphylococcus aureus), Porphyromonas gingivalis (P.g., ATCC33277) and Streptococcus mutans (M.s., ATCC25175) were purchased from Chinese strain resources Library preservation center, stored at -80°C with glycerol preservation method. Before the experiment, Escherichia coli was inoculated in LB broth medium (Beijing Suo Lai Bao Technology Co., Ltd.), Staphylococcus aureus was inoculated in TSB broth medium (Beijing Suo Lai Bao Technology Co., Ltd.), and gingival porphyri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com