Tanshinone IIA derivate comprising polyethylene glycol group and preparation and application of tanshinone IIA derivate
A technology of ethylene glycol-based double salvia and triethylene glycol-based double salvia, applied in the field of medicine, can solve problems such as low bioavailability, achieve significant anti-cancer activity, and significantly treat cardiovascular and cerebrovascular diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
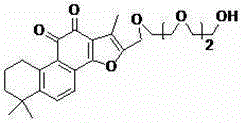
[0017] Preparation of ethylene glycol-based bistanshinone IIA (1)
[0018] The structure of compound (1) is shown below:
[0019]
[0020] Tanshinone IIA (2.94g, 10mmol) was dissolved in 15mL redistilled DMF, after cooling in an ice bath, POCl was added dropwise 3 (2.28g, 15mmol), remove the ice bath, react overnight at room temperature, after the reaction is complete, pour into 30mL ice water, extract with ethyl acetate 15mLх3, water 15mLх2, NaHCO 3 Solution 15mLх2, saturated saline 15mLх2 washing, anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by silica gel column chromatography to obtain 16-formyltanshinone IIA with a yield of 45%.
[0021] 16-Formyltanshinone IIA (3.22g, 10mmol) was dissolved in 25mL methanol and 12mL dichloromethane mixed solvent, under ice cooling, add NaBH 4 (0.76g, 20mmol), reacted for 20 minutes, slowly added 12mL of water, aliquoted methanol and dichloromethane, the aqueous layer was extracted with ethyl acetate 10mLх3, anhydrous Na ...
Embodiment 2
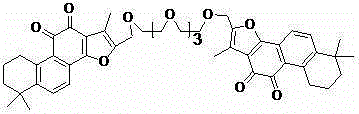
[0024] Preparation of Diethylene Glycol Bistanshinone IIA (2)
[0025] The structure of compound (2) is shown below:
[0026]
[0027] 16-Hydroxymethyltanshinone IIA (3.24g, 10mmol) was dissolved in 20mL of anhydrous dichloromethane, diethylene glycol (0.63g, 6mmol) was added, and other operations were the same as in Example 1 to obtain compound (2), Yield 43%. 1 HNMR (400MHz, CDC1 3 )δ: 7.60(d, J =8.1Hz,2H), 7.46(d, J =8.1Hz, 2H), 5.07(s, 4H), 3.62(t, J =6.2Hz,8H),3.05(m,4H),2.20(s,6H),1.77-1.79(m,4H),1.63-1.66(m,4H),1.29(s,12H).
Embodiment 3
[0029] Preparation of Triethylene Glycol Bistanshinone IIA (3)
[0030] The structure of compound (3) is shown below:
[0031]
[0032] 16-Hydroxymethyltanshinone IIA (3.24g, 10mmol) was dissolved in 20mL of anhydrous dichloromethane, triethylene glycol (0.90g, 6mmol) was added, and other operations were the same as in Example 1 to obtain compound (3), Yield 40%. 1 HNMR (400MHz, CDC1 3 )δ: 7.60(d, J =8.1Hz,2H), 7.46(d, J =8.1Hz, 2H), 5.07(s, 4H), 3.61(t, J =6.2Hz,12H),3.05(m,4H),2.20(s,6H),1.77-1.79(m,4H),1.63-1.66(m,4H),1.29(s,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com