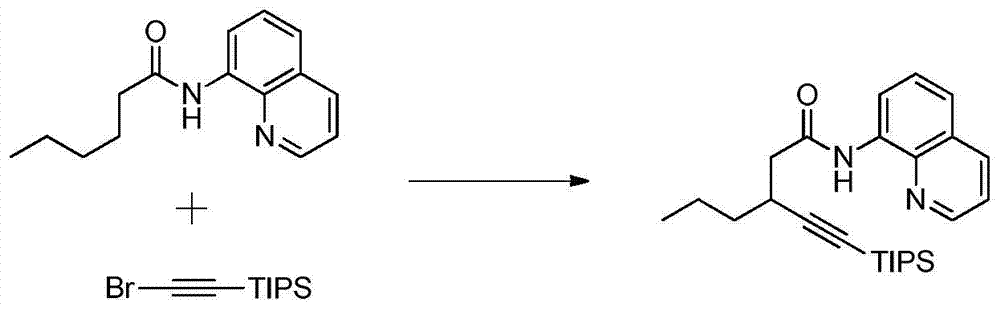
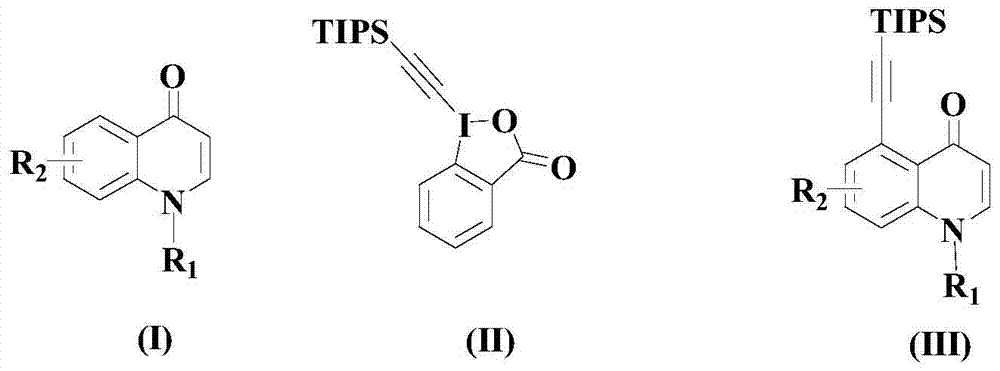
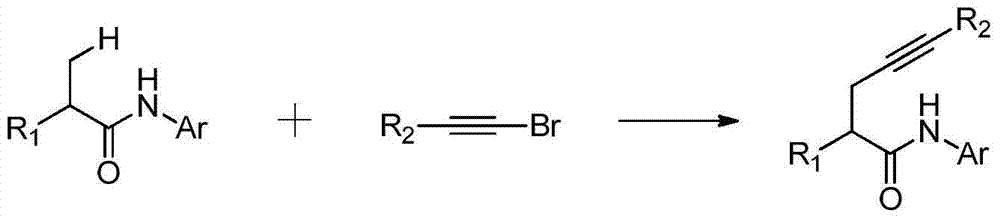A kind of synthetic method of alkyne-substituted quinolinone compound of pharmaceutical intermediate
A synthesis method and technology of quinolinones, applied in the synthesis of quinolinones and the synthesis of alkynyl-substituted quinolinones, can solve problems such as few reports, and achieve good application prospects and industrial production value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] In an appropriate amount of organic solvent (being the mixture of NMP and PEG-200 whose volume ratio is 5:1) in the reactor at room temperature, add 100mmol of the compound of formula (I) above and 100mmol of the compound of formula (II) above, and stir and mix for 10 minutes, then 3 mmol of catalyst (to be 2.4 mmol [RhCp*Cl 2 ] 2 with 0.6mmolAgBF 4 mixture) and 8mmol of auxiliary cucurbit[7]uril, heated to 50°C and kept stirring for 8 hours; after the reaction was completed, the reaction mixture was filtered, deionized water was added to the filtrate and fully shaken, and extracted with dichloromethane for 2-3 Second, the organic phases were combined, distilled under reduced pressure, and the residue was subjected to silica gel column chromatography to obtain the compound of formula (III) (wherein Bn is benzyl) in a yield of 96.5%.
[0036] 1 H NMR (CDCl 3 ,400MHz):δ7.66(d,J=9.3Hz,1H),7.46(d,J=7.9Hz,1H),7.41-7.27(m,3H),7.18(d,J=9.3Hz,1H) , 7.09 (ddd, J...
Embodiment 2
[0038]
[0039] In an appropriate amount of organic solvent (being a mixture of NMP and PEG-200 with a volume ratio of 5:1) in the reactor at room temperature, add 100mmol of the compound of the above formula (I) and 120mmol of the compound of the above formula (II), and stir and mix for 12 minutes, then 4 mmol of catalyst (to be 3.2 mmol [RhCp*Cl 2 ] 2 with 0.8mmolAgBF 4 mixture) and 12mmol of auxiliary cucurbit[7]uril, heated to 55°C and kept stirring for 7 hours; after the reaction was completed, the reaction mixture was filtered, deionized water was added to the filtrate and fully shaken, and extracted with dichloromethane for 2-3 Second, the organic phases were combined, distilled under reduced pressure, and the residue was subjected to silica gel column chromatography to isolate the compound of formula (III) (wherein Bn was benzyl) with a yield of 96.3%.
[0040] 1 H NMR (CDCl 3 ,400MHz):δ7.44(d,J=7.8Hz,1H),7.34-7.23(m,3H),7.19-7.07(m,2H),7.02(dd,J=7.8,1.8Hz,2H), 6...
Embodiment 3
[0042]
[0043] In an appropriate amount of organic solvent (being a mixture of NMP and PEG-200 with a volume ratio of 5:1) in the reactor at room temperature, add 100mmol of the compound of formula (I) above and 150mmol of the compound of formula (II) above, and stir and mix for 15 minutes, then add 5 mmol of catalyst (to be 4 mmol [RhCp*Cl 2 ] 2 with 1mmolAgBF 4 mixture) and 10mmol of auxiliary cucurbit[7]uril, heated to 60°C and kept stirring for 6 hours; after the reaction was completed, the reaction mixture was filtered, deionized water was added to the filtrate and fully shaken, and extracted with dichloromethane for 2-3 For the second time, the organic phases were combined, distilled under reduced pressure, and the residue was subjected to silica gel column chromatography to isolate the compound of the above formula (III) with a yield of 96.2%.
[0044] 1 H NMR (CDCl 3 ,400MHz):δ7.57-7.43(m,2H),7.29(d,J=7.7Hz,1H),7.24(dd,J=8.3,1.5Hz,1H),6.13(d,J=7.7Hz, 1H), 3.72...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com