Preparation method of O-carboxymethyl chitosan
A technology of carboxymethyl chitosan and chitosan, which is applied in the field of preparation of O-carboxymethyl chitosan, can solve the problems of being susceptible to temperature fluctuations, and achieves accelerated wound healing, good adsorption effect, and strong load capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
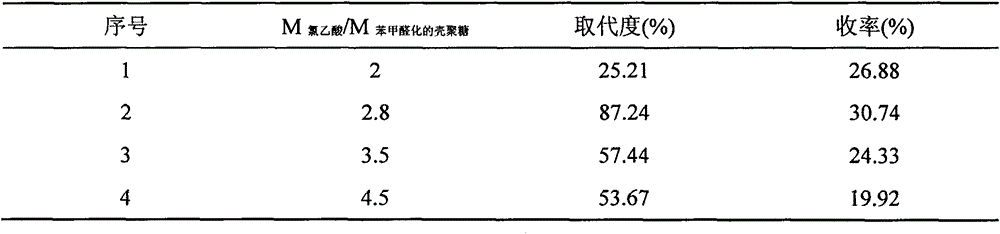
[0012] (1) Chitosan C 2 Protection of amino groups: Accurately weigh 3.0g of CTS and dissolve in 240ml of 1% acetic acid solution, dissolve completely at 25°C and slowly add 180ml of methanol. Accurately weigh 3.0g benzaldehyde and dissolve it in 45ml methanol, mix evenly and add dropwise, heat up to 60°C and stir for 4h. Slowly add 240ml of 5% NaOH solution and 60ml of methanol, and continue stirring for 8h. After the reaction, the precipitate was filtered by suction, washed with 95% ethanol and dehydrated with absolute ethanol to remove unreacted benzaldehyde, and dried at 55°C. Respond many times to accumulate the product for later use.
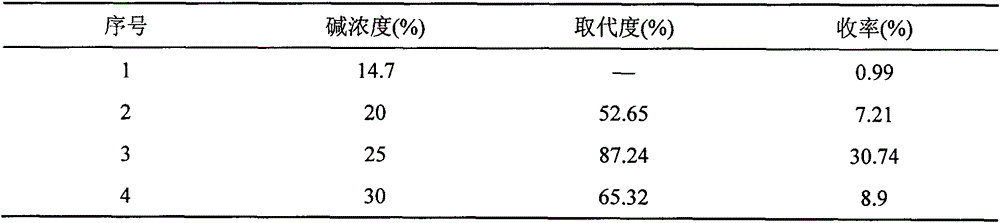
[0013] (2) Carboxymethyl reaction: Take 5.0 g of chitosan after the above reaction (passed through a 60-mesh sieve) and suspend it in 50 ml of isopropanol, and swell at 55° C. for 5 hours. Cool to 25°C, and slowly add 25ml of 20% NaOH solution dropwise with stirring at a rate of 3 s / d. After the dropwise addition, continue stirring for...
Embodiment 2
[0016] (1) Chitosan C 2 Protection of amino groups: Accurately weigh 3.0g of CTS and dissolve in 240ml of 1% acetic acid solution, dissolve completely at 25°C and slowly add 180ml of methanol. Accurately weigh 3.0g benzaldehyde and dissolve it in 45ml methanol, mix evenly and add dropwise, heat up to 60°C and stir for 4h. Slowly add 240ml of 5% NaOH solution and 60ml of methanol, and continue stirring for 8h. After the reaction, the precipitate was filtered by suction, washed with 95% ethanol and dehydrated with absolute ethanol to remove unreacted benzaldehyde, and dried at 55°C. Respond many times to accumulate the product for later use.
[0017] (2) Carboxymethyl reaction: Take 5.0 g of chitosan after the above reaction (passed through a 60-mesh sieve) and suspend it in 50 ml of isopropanol, and swell at 55° C. for 5 hours. Cool to 25°C, slowly add 25ml of 25% NaOH solution dropwise with stirring at a rate of 3 s / d. After the dropwise addition, continue stirring for 1 h...
Embodiment 3
[0020] (1) Chitosan C 2 Protection of amino groups: Accurately weigh 3.0g of CTS and dissolve in 240ml of 1% acetic acid solution, dissolve completely at 25°C and slowly add 180ml of methanol. Accurately weigh 3.0g benzaldehyde and dissolve it in 45ml methanol, mix evenly and add dropwise, heat up to 60°C and stir for 4h. Slowly add 240ml of 5% NaOH solution and 60ml of methanol, and continue stirring for 8h. After the reaction, the precipitate was filtered by suction, washed with 95% ethanol and dehydrated with absolute ethanol to remove unreacted benzaldehyde, and dried at 55°C. Respond many times to accumulate the product for later use.
[0021] (2) Carboxymethyl reaction: Take 5.0 g of chitosan after the above reaction (passed through a 60-mesh sieve) and suspend it in 50 ml of isopropanol, and swell at 55° C. for 5 hours. Cool to 25°C, slowly add 25ml of 25% NaOH solution dropwise with stirring at a rate of 3 s / d. After the dropwise addition, continue stirring for 1 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com