A dronedarone hydrochloride composition
A technology of dronedarone hydrochloride and dronedarone hydrochloride tablets, applied in the field of medicine, can solve problems such as occurrence of splits, fragments, influence on drug safety and effective use, etc., and achieve the effect of convenient transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
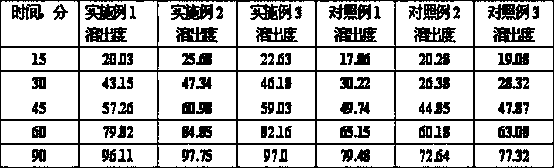
Embodiment 1
[0024] Example 1: Dronedarone Hydrochloride 400g, Sodium Lauryl Sulfate 2g, Lactose 90g, Hypromellose 16.8g, Crospovidone 10g, Silicon Dioxide 6.48g, Magnesium Stearate 6.48g , prepare 1000 tablets as follows.
[0025] (1) Co-micronize dronedarone hydrochloride and sodium lauryl sulfate with a jet mill, the particle size D90 after micronization is 5um, and set aside.
[0026] (2) Weigh the prescribed amount of hypromellose and add it into the prescribed amount of purified water to prepare a 7% aqueous solution of hypromellose (W / W), stir and dissolve until evenly mixed, and set aside.
[0027] (3) Weigh the co-micronized material and other auxiliary materials prepared in the first step according to the specification ratio, put them into the one-step granulator, turn on the fan and heat to make the material evenly heated in the fluidized state, turn on the spray button, and spray into the viscous Mixture, adjust the liquid inlet speed, atomization pressure, inlet air temperatu...
Embodiment 2
[0030] Example 2: 400 g of dronedarone hydrochloride, 5 g of polyethylene glycol 4000, 120 g of lactose, 16.8 g of hydroxypropylmethylcellulose, 10 g of crospovidone, 6.41 g of silicon dioxide, and 6.41 g of magnesium stearate. According to Example 1, dronedarone hydrochloride and polyethylene glycol 4000 were co-micronized, and the particle size D90 after micronization was 10 um. The preparation method prepared 1000 tablets.
Embodiment 3
[0031] Example 3, Dronedarone Hydrochloride 400g, Sodium Lauryl Sulfate 3g, Lactose 100g, Crospovidone 10g, Hypromellose 16.8g, Silicon Dioxide 6.4g, Magnesium Stearate 6.4g According to the preparation method of Example 1, dronedarone hydrochloride and sodium lauryl sulfate were micronized, and the particle size D90 after micronization was 8um, and 1000 tablets were prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com