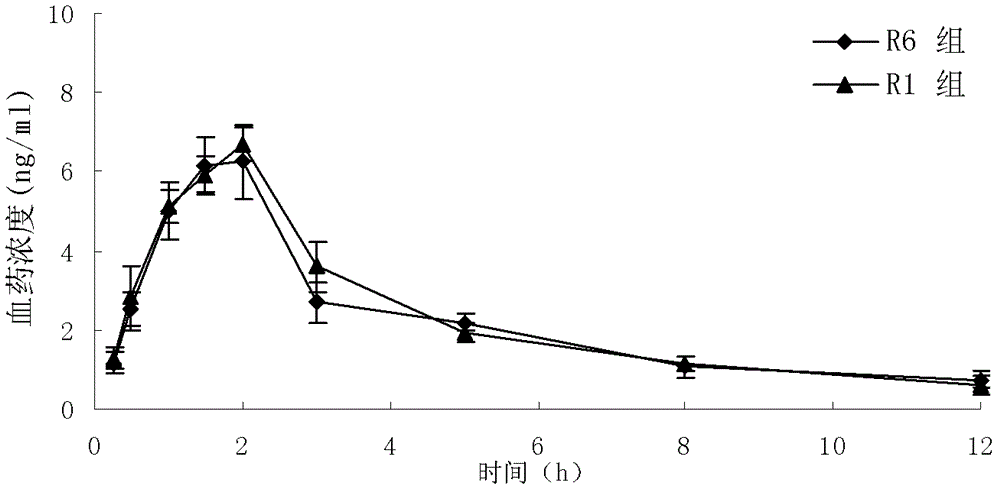
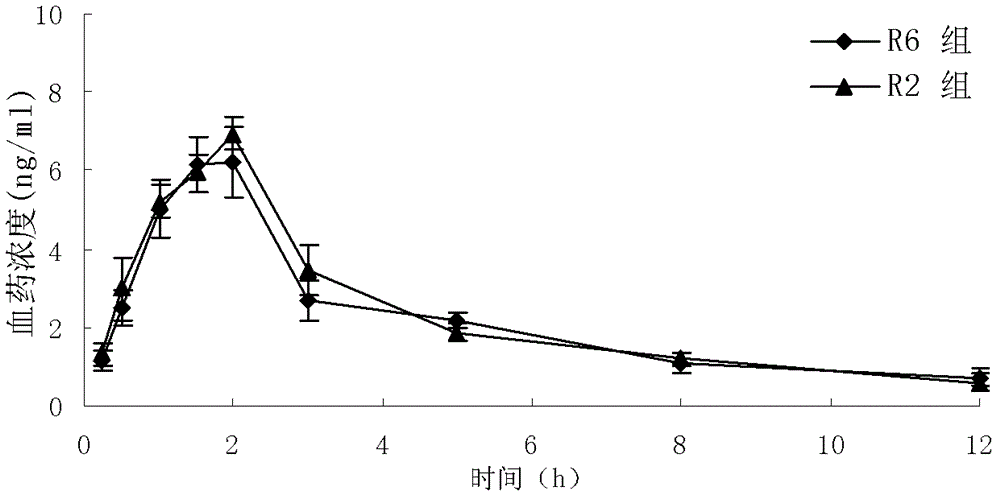
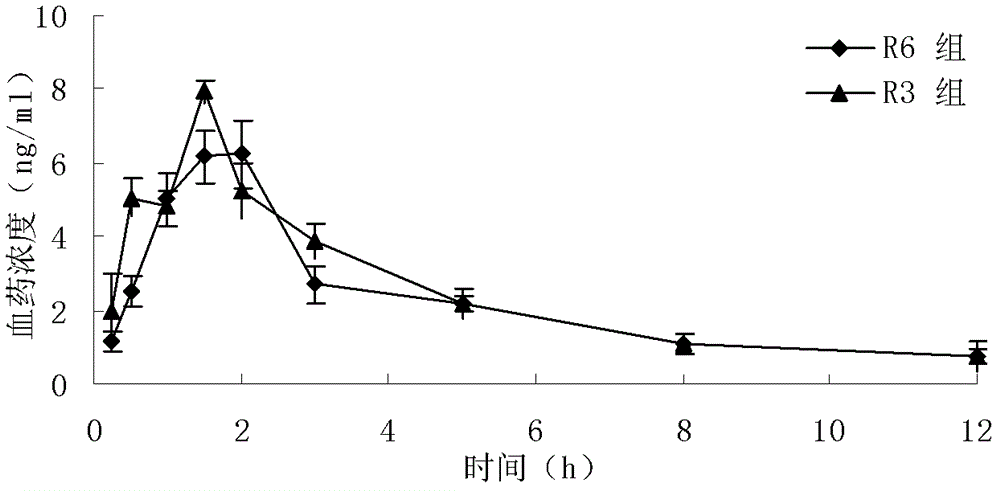
Granisetron hydrochloride freeze-dried tablets and preparation method thereof
A technology of granisetron hydrochloride and freeze-dried tablets, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of low bioavailability of granisetron hydrochloride, difficulty in drying, obvious side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] The preparation formula of the present invention is made up of following components:
[0122]
[0123]
[0124] A total of 1000 pieces were made
[0125] The specific preparation method is as follows: mix granisetron hydrochloride, glycine, pullulan, acesulfame potassium, and pineapple essence evenly, add the purified water of the prescribed amount to it, and mix to form a homogeneous solution; vacuum degas the solution Finally, pour it into the mold accurately; pre-freeze at -40°C to -170°C for 1 to 60 minutes, then transfer to a freeze dryer, and freeze dry at a pressure of 0.01mbar to 10mbar and at a temperature of -30°C to 30°C 1~10h, obtain the granisetron hydrochloride freeze-dried tablet of the present invention; In the above-mentioned method, after the pre-freezing step, the step of ice crystal incubation can also be added before being transferred to the lyophilizer, that is, the mold that is filled with the solution after the pre-freezing, put Into the l...
Embodiment 2
[0127] The preparation formula of the present invention is made up of following components:
[0128]
[0129] A total of 1000 pieces were made
[0130] The specific preparation method is as follows: mix the granisetron hydrochloride, mannitol, pullulan, aspartame, and sweet orange essence in the above-mentioned dosages evenly, add the purified water of the prescribed amount to it, and mix to form a homogeneous solution; All the other preparation methods are the same as in Example 1.
Embodiment 3
[0132] The preparation formula of the present invention is made up of following components:
[0133]
[0134] A total of 1000 pieces were made
[0135] The specific preparation method is as follows: mix the granisetron hydrochloride, glycine, sodium alginate, and sucralose in the above-mentioned amounts evenly, add the purified water of the prescribed amount to it, and mix to make a homogeneous solution; the rest of the preparation methods are the same as in the examples 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com