Crystal form and preparation method of cyclin-dependent kinase inhibitor
A crystal form and compound technology, applied in the field of chemical medicine, can solve the problems of low humidity stability of the monosuccinate crystal form, easy conversion into other crystal forms, unfavorable drug development and storage, etc., to simplify the preparation and post-treatment process , Facilitate absorption and utilization, reduce material storage and quality control costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation method of hemisuccinate crystal form A:
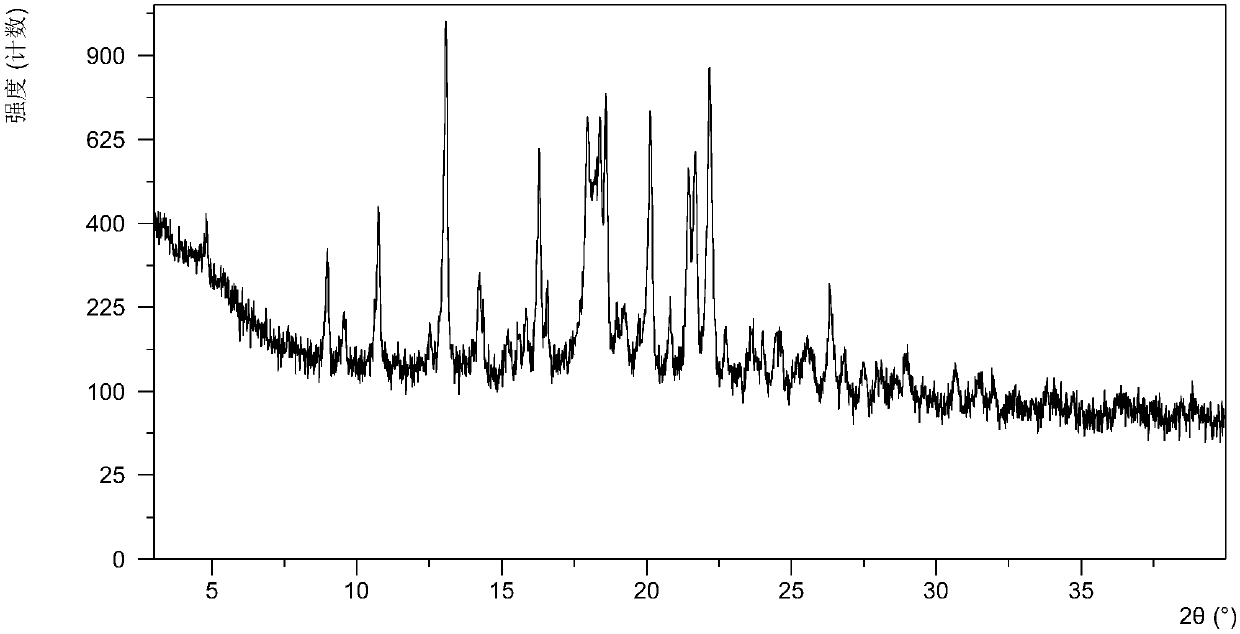
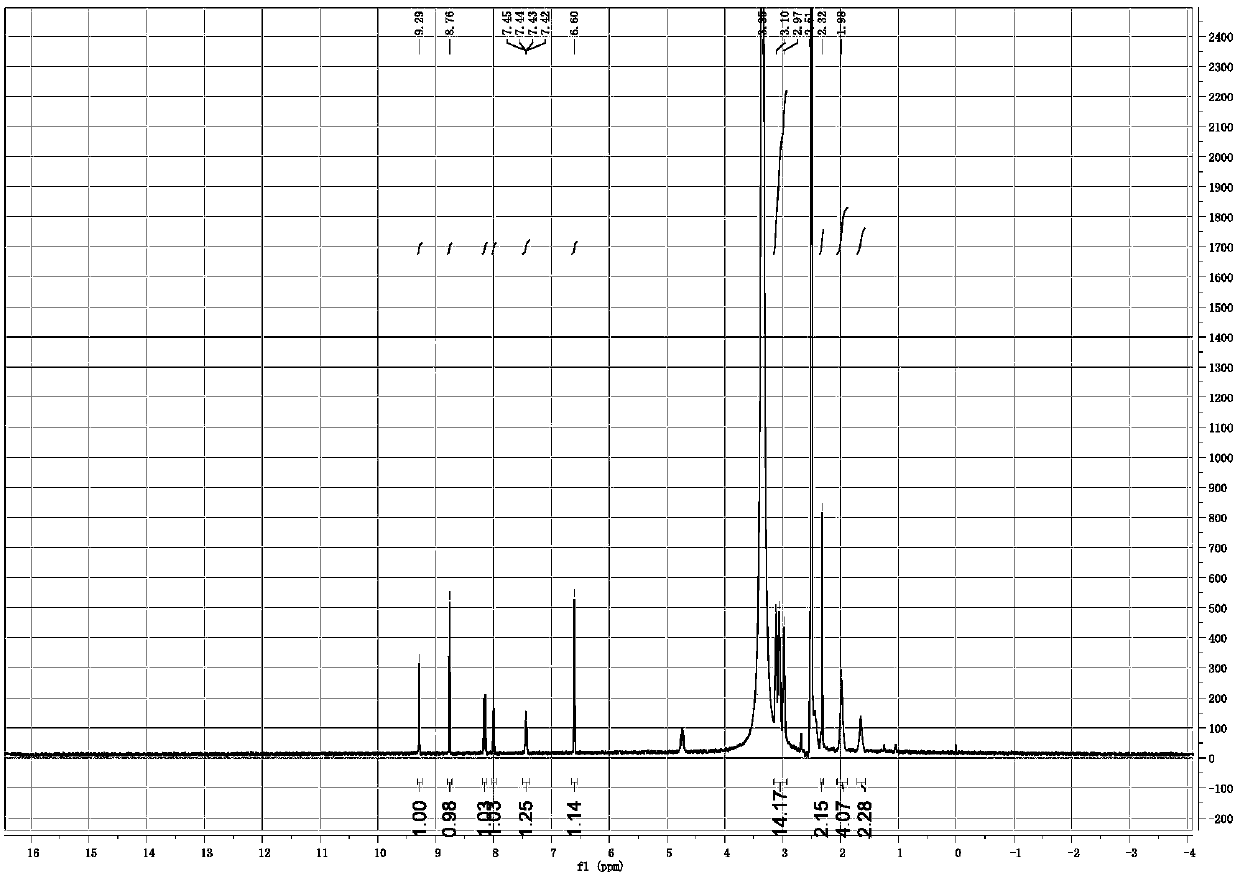
[0073] Dissolve 10.5 mg of the free base of the compound of formula (I) in ethanol, add 3.0 mg of succinic acid, stir at room temperature for 12 hours, and obtain crystallization. The crystal form A that present embodiment obtains passes through XRPD, DSC, TGA and 1 HNMR detection. Its X-ray powder diffraction data are shown in Table 1. DSC data showed that an endothermic peak appeared when heated to 180°C, and TGA data showed a 12.5% weight loss when heated from room temperature to 118°C. Its XRPD pattern is as follows figure 1 , 1 HNMR picture as shown figure 2 .
[0074] The compound hemisuccinate of formula (I) prepared by the above-mentioned method, its 1 HNMR identification data are as follows:
[0075] 1 HNMR (400MHz, DMSO) δ9.29(s, 1H), 8.76(s, 1H), 8.16(d, J=9.0Hz, 1H), 8.00(d, J=2.9Hz, 1H), 7.44(dd, J=9.2,3.0Hz,1H),6.60(s,1H),3.15–2.93(m,14H),2.32(s,2H),1.98(s,4H),1.65(s,2H).
[0076] Tabl...
Embodiment 2
[0079] The preparation method of hemisuccinate crystal form A:
[0080] Dissolve 10.2 mg of the free base of the compound of formula (I) in tetrahydrofuran, add 2.8 mg of succinic acid and stir at room temperature for 12 hours to obtain crystallization. Table 2 shows the X-ray powder diffraction data of Form A obtained in this example.
[0081] Table 2
[0082] 2theta
[0083] 33.72
Embodiment 3
[0085] Stability study of hemisuccinate form A under high humidity conditions:
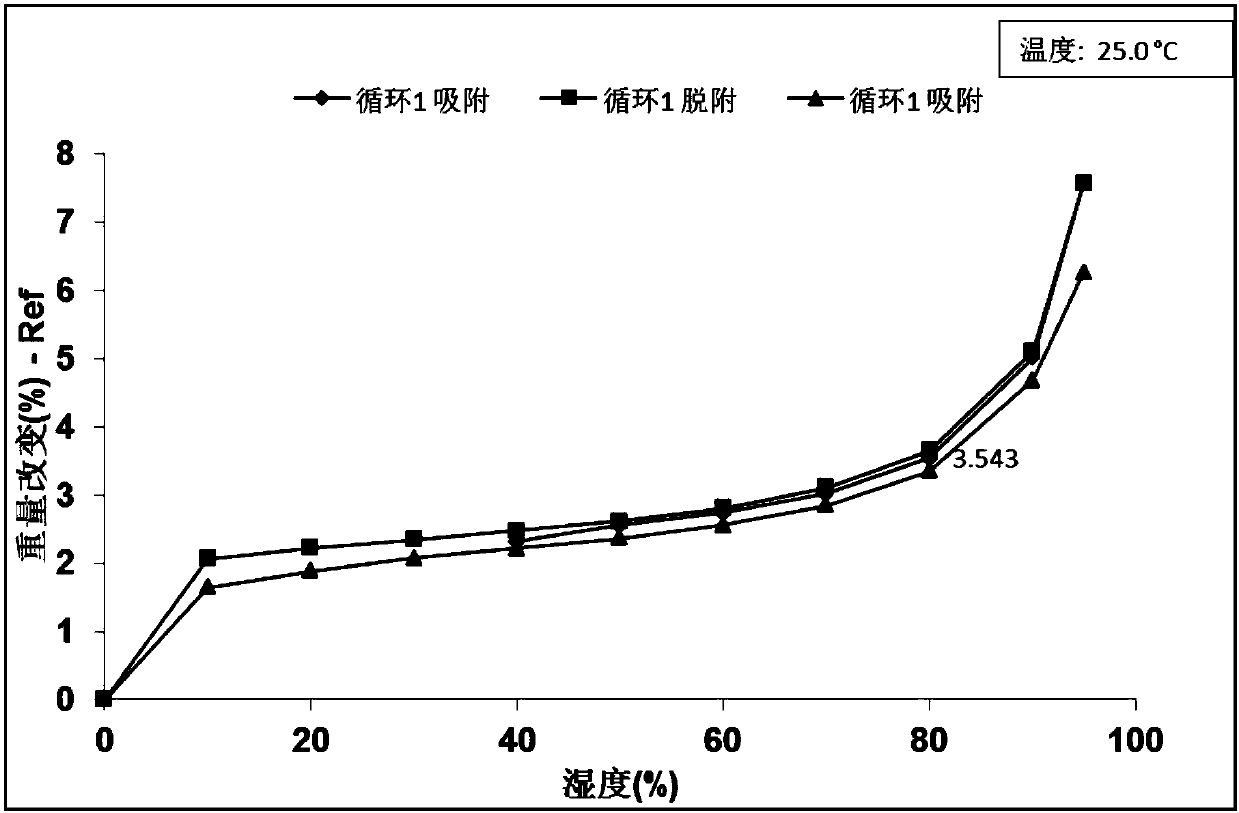
[0086] Take about 10 mg of the crystal form A of the present invention to perform a dynamic moisture adsorption test with a dynamic moisture adsorption (DVS) instrument, and detect XRPD before and after the test. The results show that the crystal form A of the present invention has a weight gain of 3.543% at 80% relative humidity, and its hygroscopicity is low, and its DVS diagram is as follows image 3 shown. The crystal form remains unchanged before and after the dynamic water adsorption test, and its XRPD comparison chart is as follows Figure 4 shown.
[0087] Regarding the description of hygroscopic characteristics and the definition of hygroscopic weight gain (Chinese Pharmacopoeia 2010 edition appendix XIXJ drug hygroscopicity test guidelines, experimental conditions: 25 ° C ± 1 ° C, 80% relative humidity):
[0088] Deliquescence: the absorption of sufficient water to form a liquid
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com