Drug-sustained-releasing type corneal contact lens hydrogel material, preparing method and application
A technology of corneal contact lens and hydrogel, which is applied in the field of biomedical materials, can solve the problems of limited drug absorption ability of contact lens, can not meet the needs of practical application, and low water absorption rate of pHEMA, so as to prevent dry eye syndrome, which is of great significance and The effect of suppressing inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
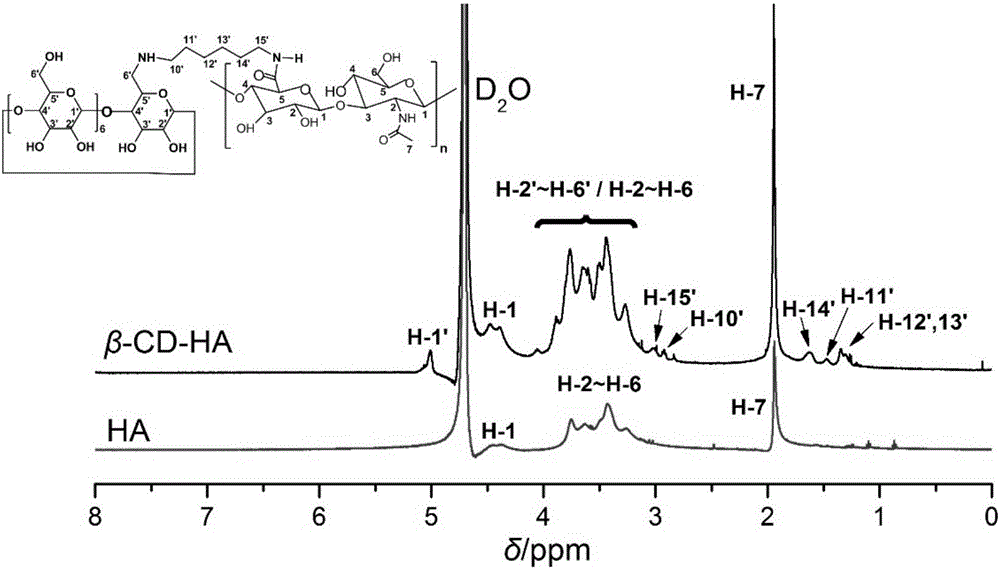
Examples
Embodiment 1
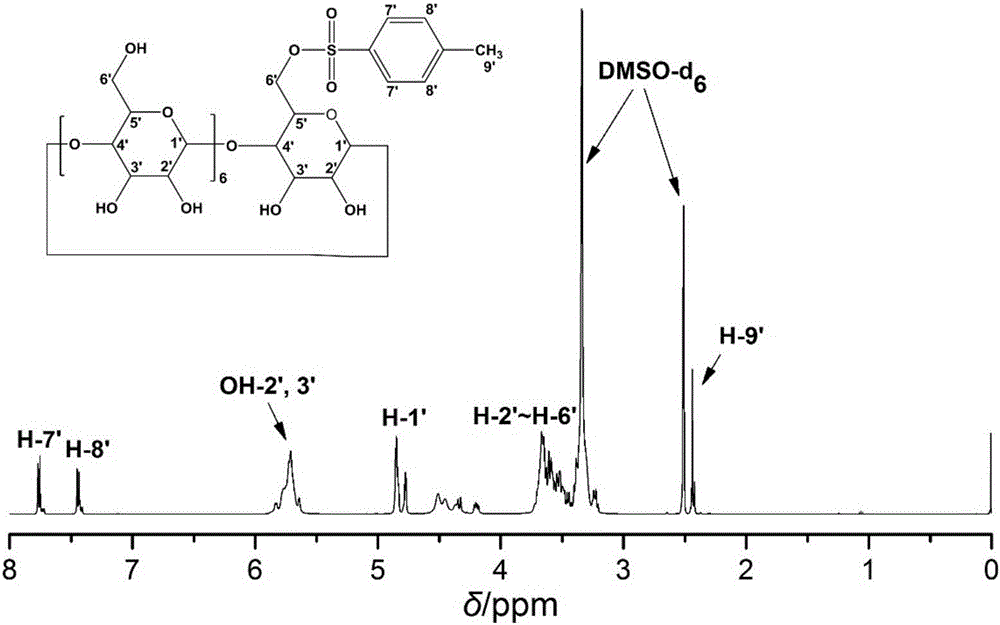
[0059] (1) Slowly add 20 mL of 0.33 g / mL NaOH solution dropwise to 500 mL of β-CD suspension with a concentration of 0.12 g / mL, and the suspension slowly becomes clear; add 30 mL of paraformaldehyde with a concentration of 0.34 g / mL The acetonitrile solution of phenylsulfonyl chloride (p-TsCl) was added dropwise to the above clarified solution, and a white precipitate was gradually formed, and then the reaction was stirred for 2 h; the unreacted p-TsCl was separated by suction filtration under reduced pressure, and the filtrate was placed in 4 After standing in the refrigerator for 48h, a large amount of white precipitate was precipitated; the precipitate was collected by vacuum filtration again, and dried in vacuum for 48h to obtain the product Mono-6-O-Ts-β-CD;
[0060] in, figure 1 for Mono-6-O-Ts-β-CD 1 HNMR spectra, such as figure 1 As shown in the middle spectrum, the proton peaks of Mono-6-O-Ts-β-CD are assigned as follows: 2.42ppm (H-9'), 3.24~3.67ppm (H-2'~H-6'), 4....
Embodiment 2
[0068] (1) Slowly add 20 mL of 0.33 g / mL NaOH solution dropwise to 500 mL of β-CD suspension with a concentration of 0.12 g / mL, and the suspension slowly becomes clear; add 30 mL of paraformaldehyde with a concentration of 0.34 g / mL The acetonitrile solution of phenylsulfonyl chloride (p-TsCl) was added dropwise to the above clarified solution, and a white precipitate was gradually formed, and then the reaction was stirred for 4 h; the unreacted p-TsCl was separated by suction filtration under reduced pressure, and the filtrate was placed in 4 After standing in the refrigerator for 48h, a large amount of white precipitate was precipitated; the precipitate was collected by vacuum filtration again, and dried in vacuum for 48h to obtain the product Mono-6-O-Ts-β-CD;
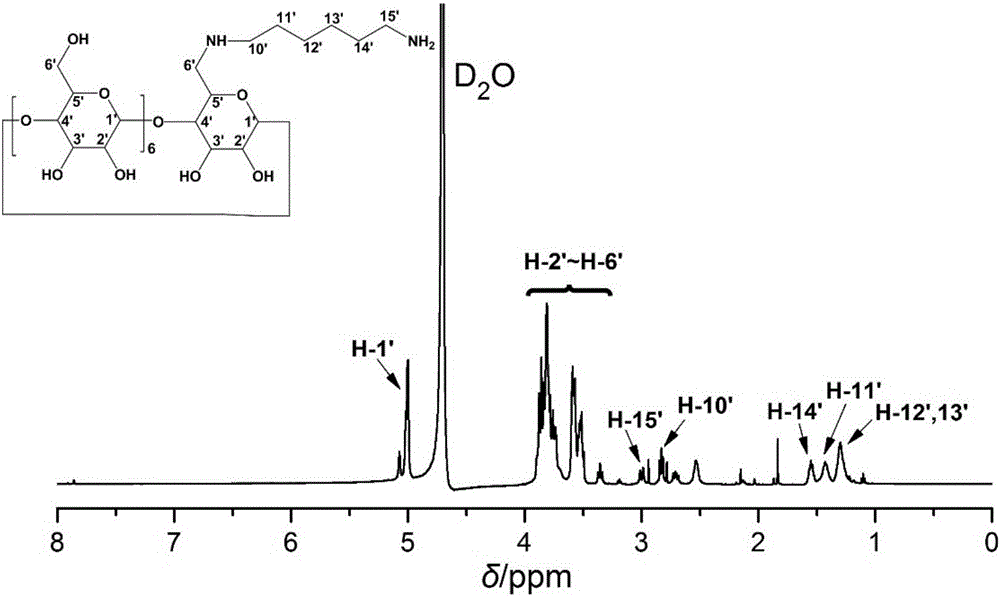
[0069] (2) Dissolve 2.26 mmol of Mono-6-O-Ts-β-CD obtained in step (1) and 51.6 mmol of hexamethylenediamine (HDA) in 12 mL of dimethylformamide, and stir and react at 60°C for 8 h, Then an excess of cold acetone wa...
Embodiment 3
[0073] (1) Slowly add 20 mL of 0.33 g / mL NaOH solution dropwise to 500 mL of β-CD suspension with a concentration of 0.12 g / mL, and the suspension slowly becomes clear; add 30 mL of paraformaldehyde with a concentration of 0.34 g / mL The acetonitrile solution of phenylsulfonyl chloride (p-TsCl) was added dropwise to the above clear solution, and a white precipitate gradually formed. Then the reaction was stirred for 8 hours; the unreacted p-TsCl was separated by suction filtration under reduced pressure, and the filtrate was placed in a refrigerator at 4 °C for 48 hours, and a large amount of white precipitate was precipitated; The product Mono-6-O-Ts-β-CD was obtained;
[0074] (2) Dissolve 2.26 mmol of Mono-6-O-Ts-β-CD obtained in step (1) and 51.6 mmol of hexamethylenediamine (HDA) in 12 mL of dimethylformamide, and stir and react at 80°C for 2 h, Then an excess of cold acetone was added to separate out a white precipitate, and the white precipitate was isolated by suction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com