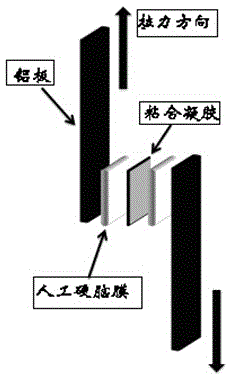
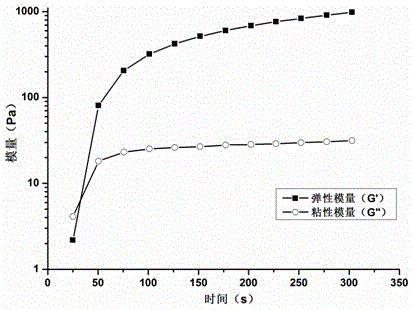
Medical injectable bonding gel and preparation method thereof
A gel and injection device technology, applied in the fields of application, medical science, surgical adhesives, etc., can solve the problems of unsuitable mass production of oxidized dextran, high consumption, and long dialysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of modified dextran (molecular weight 1200KDa, oxidation degree 20%)
[0048] Weigh 250 g of dextran powder (containing 1.54 mol of glucose units, molecular weight of 1200 KDa), and dissolve it in 2.5 L of deionized water to obtain an aqueous solution of dextran. Under dark conditions, weigh 66 g of sodium periodate (0.31 mol, accounting for 20% of the total glucose units), and dissolve it in 0.2 L of deionized water. The sodium periodate solution was mixed with the dextran solution, the temperature of the water bath was controlled to be 30° C., and the reaction was continued for 3 h in a dark environment. After the reaction, the reaction solution was first passed through an anion exchange column containing 7L strong base anion resin (the material is strong base polystyrene gel resin, Tianjin Nankai Hecheng Technology Co., Ltd., model 001×7), and then passed through the same Volumetric cation exchange column (material is strong acid polystyrene g...
Embodiment 2
[0049] Example 2: Preparation of modified dextran (molecular weight 800KDa, degree of oxidation 100%)
[0050] Weigh 250 g of dextran powder (containing 1.54 mol of glucose units, molecular weight 800 KDa), and dissolve it in 2.0 L of deionized water to obtain an aqueous solution of dextran. Under dark conditions, weigh 395 g of sodium periodate (1.85 mol, accounting for 120% of the total glucose units), and dissolve it in 1 L of deionized water. The sodium periodate solution was mixed with the dextran solution, the temperature of the water bath was controlled to be 30° C., and the reaction was continued for 3 h in a dark environment. Add 40 mL (0.55 mol) of glycerol to quench unreacted sodium periodate. After the reaction is over, pass the reaction solution through an anion exchange column containing 7L strong base anion resin (the same exchange column as described above), and then pass through a cation exchange column of the same volume (same as the exchange column describe...
Embodiment 3
[0051] Example 3: Preparation of medical injectable adhesive gel (modified dextran molecular weight 1000KDa, oxidation degree 50%)
[0052] Weigh 250 g of dextran powder (containing 1.54 mol of glucose units, molecular weight of 1000 KDa), and dissolve it in 2.0 L of deionized water to obtain an aqueous solution of dextran. Under dark conditions, weigh 165 g of sodium periodate (0.77 mol, accounting for 50% of the total glucose units), and dissolve it in 0.8 L of deionized water. The sodium periodate solution was mixed with the dextran solution, the temperature of the water bath was controlled to be 30° C., and the reaction was continued for 3 h in a dark environment. After the reaction, the reaction solution was first passed through an anion exchange column containing 7L strong base anion resin, and then passed through a cation exchange column with the same volume, and the flow rate was controlled at 50mL min -1 . Detect that the sodium iodate content in the effluent is les...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com