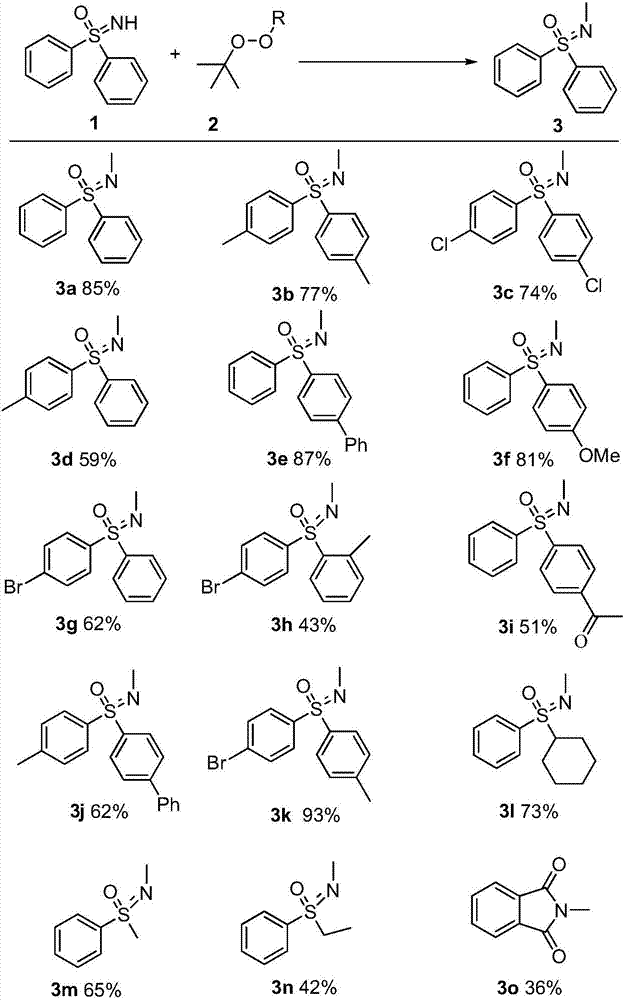
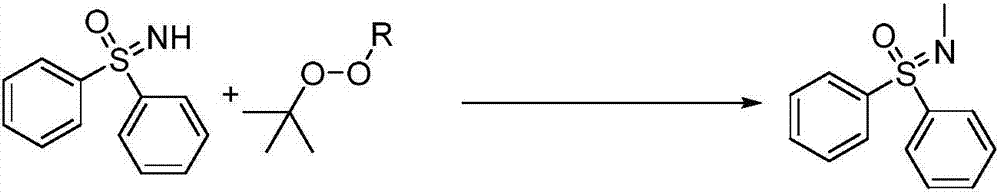Synthetic method of novel n-methylated sulfoximine derivatives
A technology of methylated sulfoximine and diphenyl sulfoximine, which is applied in the field of synthesis of N-methylated sulfoximine derivatives, can solve problems such as long reaction time, and achieve short reaction time and high processing efficiency. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Mix diphenylsulfoximine 1a (2 mmol), tert-butanol peroxide (4 mmol) and copper chloride (0.2 mmol), add 20 mL of chlorobenzene as a solvent, and react under heating for 4 hours. The conversion of diphenylsulfoximine was 96%, and the yield of 3a was 85%.
Embodiment 2
[0022] Mix 4,4'-dimethyldiphenylsulfoximine 1b (2mmol), dicumyl peroxide (1mmol) and copper bromide (0.2mmol), add 20mL of fluorobenzene as a solvent, and heat reaction, the reaction time was 2 hours. The conversion of 1b was 89%, and the yield of 3b was 77%.
Embodiment 3
[0024] Mix 4,4'-dichlorodiphenylsulfoximine 1c (2mmol), tert-butyl peroxybenzoate (6mmol) and copper chloride (0.2mmol), add 20mL of toluene as a solvent, and react under heating , the reaction time was 16 hours. The conversion of 1c was 92%, and the yield of 3c was 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com