Application of quinoline-sulfamide compound as inhibitor for Th17 cell differentiation
A technology of sulfonamides and compounds is applied in the application field of quinoline-sulfonamide compounds as Th17 cell differentiation inhibitors, which can solve the problem of weakening the clinical symptoms of autoimmune diseases in mice, reducing the differentiation ability of Th17 cells, and failing to find target drugs. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
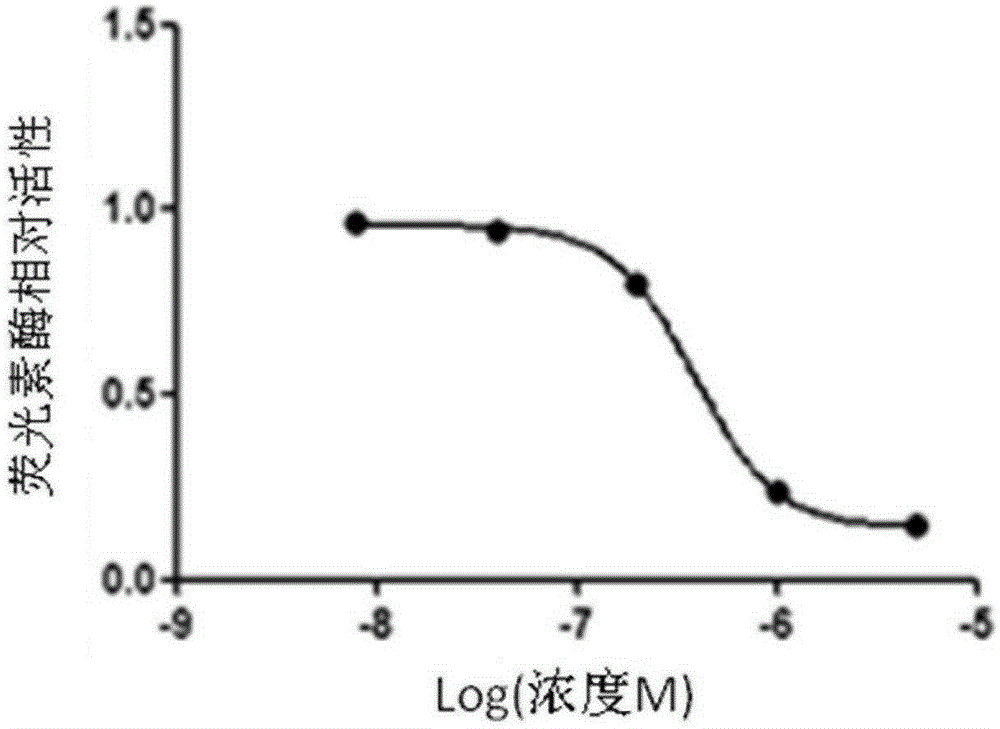
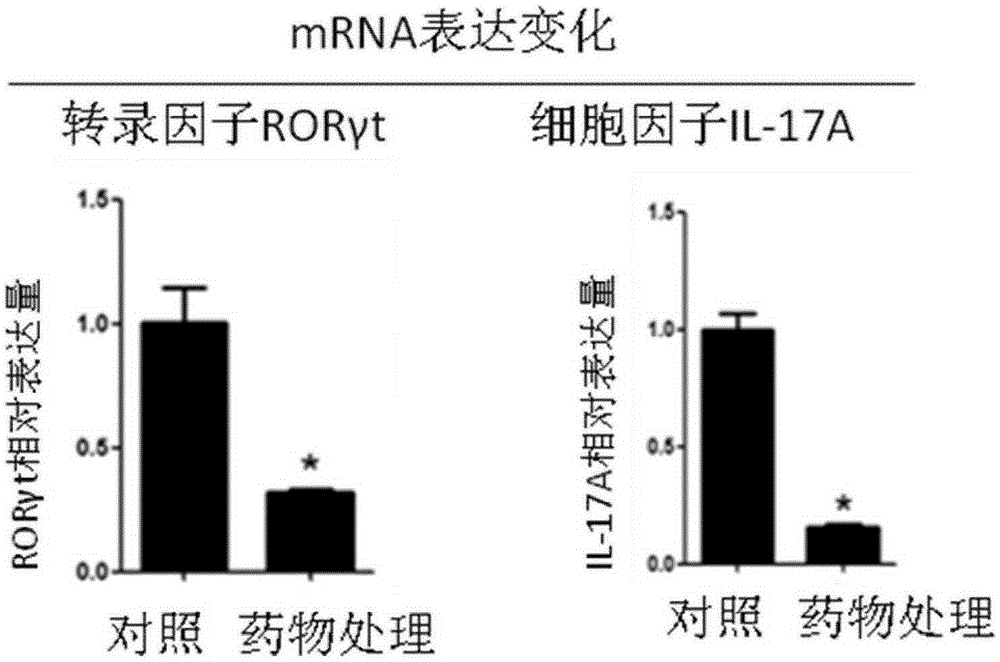
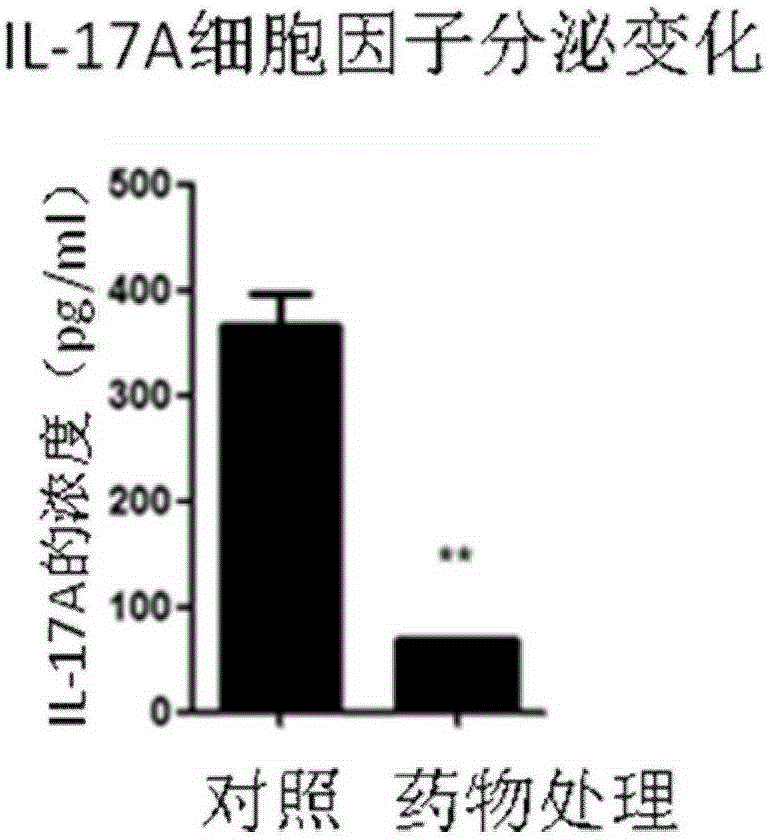
[0020] In the following experiments, the structural formula of the quinoline-sulfonamide compound is (Formula I), when the Cl element is replaced by other halogen elements fluorine (F), bromine (Br) or iodine (I), it also has a similar effect to Cl, and the experimental results will not be given here.
[0021] The screening system for luciferase activity based on transcription factor activity can be constructed according to existing methods. Or build it as follows:
[0022] Material:
[0023]The cell lines Jurkat and 293T were preserved in our laboratory; the bacterial strain DH5α was donated by Professor Ma Runlin, Institute of Genetics, Chinese Academy of Sciences, Beijing; the restriction endonuclease was purchased from Fermentas Company of the United States; the DNA ligase was purchased from NEB Company of the United States; the reporter gene sequence IRES-GFP It is preserved by the inventor (other existing reporter gene sequences can also be used); the reporter plasm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com