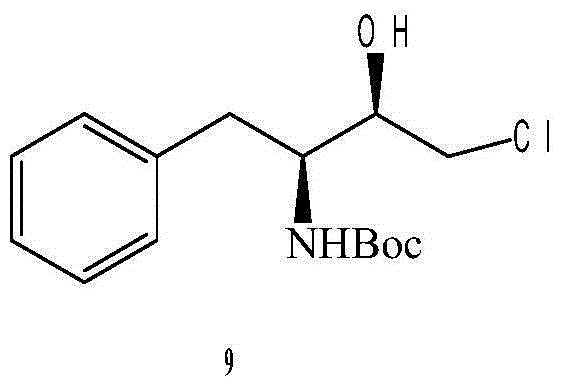
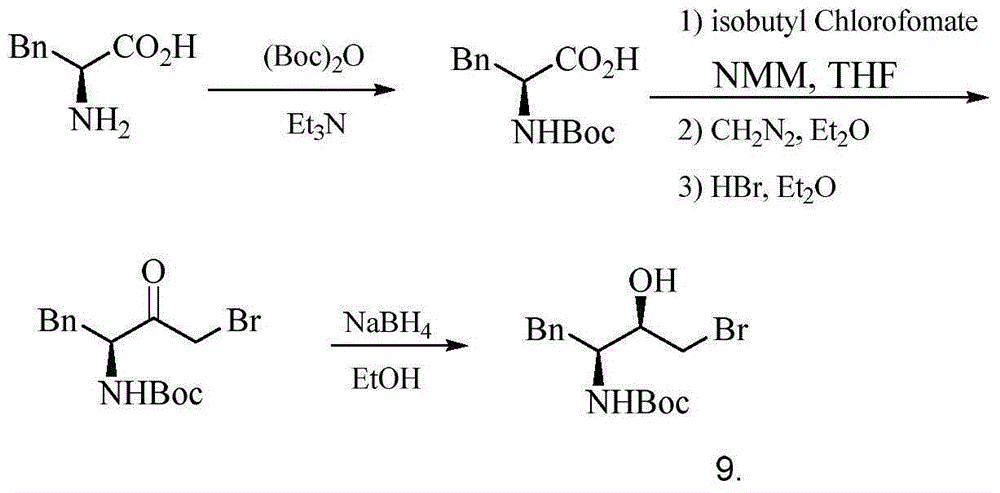
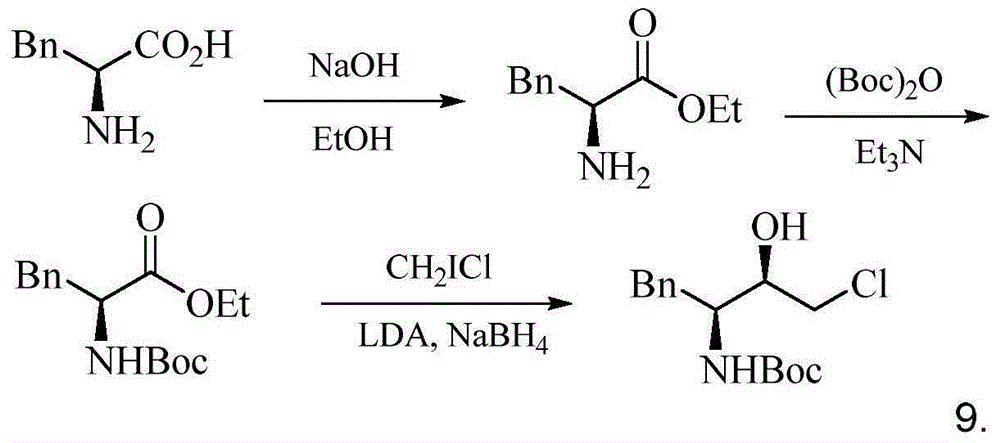
Preparation method for (2R,3S)-1-chlorine-3-tert-butoxycarbonylamino-4-phenyl-2-butanol
A technology of tert-butoxyamido and phenyl, applied in the preparation of -1-chloro-3-tert-butoxyamido-4-phenyl-2-butanol, the field of anti-AIDS drug amprenavir intermediates , can solve problems such as not suitable for industrial production, difficult separation of products, harsh reaction conditions, etc., to achieve safe yield, improve production efficiency, and realize the effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The first step: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0040] Add L-phenylalanine 1 (20.0g, 121.1mmol), K 2 CO 3 (60.0g, 434.8mmol), H 2 The mixture of O (90ml), EtOH (45ml), BnCl (45.2g, 394.7mmol) was heated to 90°C, and the reaction was stopped after 15h. After the reaction, the water layer was removed, and 100 ml of n-hexane was added to the organic layer and washed with 500 ml of water, dried, filtered, and spin-dried to obtain a pale yellow liquid compound 2 (50.6 g, 96%). 1 H-NMR (300MHz, CDCl 3 ): δ3.20(dd, 2H, J=8.4, 14.4Hz, Ph CH 2 C), 3.60(d,2H,J=15.0Hz,2Ph CHaHb N), 3.80(dd,1H,J=8.5,8.5Hz,N CH ), 4.00(d,2H,J=15.0Hz,2Ph CHaHb N), 5.20 (d, 1H, J = 13.5 Hz, Ph CHaHb O),5.30(d,1H,J=13.5Hz,Ph CHaHb O), 7.50-7.00 (m, 20H, 4PhH) ppm. 13 C-NMR(125MHz, CDCl 3 ):δ35.6,54.3,62.3,66.0,126.2,126.9,128.1,128.2,128.4,128.5,128.6,129.4,135.9,138.0,139.2,172.0ppm.MS(ESI,m / z):436.0(MH + ).
[0041] The second step: N,N-dibenzyl-L-phenylalanine 3
[00...
Embodiment 2
[0056] The first step: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0057] Dissolve L-phenylalanine 1 (50.0g, 302.7mmol), NaOH (20.0g, 500.0mmol), BnCl (294.9g, 908.7mmol) in 250ml of water and 200ml of EtOH, heat to 90°C and reflux, N 2 The reaction was carried out at this temperature for 10 hours under protection. After the reaction was completed, toluene (2×250ml) was added. Several layers were washed successively with water and saturated brine, dried, filtered, and spin-dried to obtain a pale yellow liquid compound 2 (121.3g, 92 %).
[0058] The second step: (S)-N,N-dibenzyl-L-phenylalanine 3
[0059] The same as in Example 1.
[0060] The third step: Bn-mixed anhydride 4
[0061] A 500ml glass reactor was equipped with a 250ml addition funnel, stirrer, thermometer and cooling bath. Dissolve (S)-N,N-dibenzyl-L-phenylalanine 3 (50.0g, 130.9mmol) in dichloromethane (200ml), add N-methylmorpholine at once while stirring (16.0 g), transfer the clear, colorless solut...
Embodiment 3
[0073] The first step: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0074] Add L-phenylalanine 1 (20.0g, 122.7mmol), K 2 CO 3 (60.0g, 606mmol), H 2 The mixture of O (90ml), EtOH (45ml) and BnCl (50.0g, 393.7mmol) was heated to 80°C and the reaction was stopped after 48h. After the reaction, the water layer was removed, and 100 ml of n-hexane was added to the organic layer and washed with 500 ml of water, dried, filtered, and spin-dried to obtain a pale yellow liquid compound 2 (51.7 g, 96.8%).
[0075] The second step: (S)-N,N-dibenzyl-L-phenylalanine 3
[0076] The same as in Example 1.
[0077] The third step: Bn-mixed anhydride 4
[0078] A 250ml glass reactor was equipped with a 250ml addition funnel, stirrer, thermometer and cooling bath. Dissolve (S)-N,N-dibenzyl-L-phenylalanine 3 (25.0g, 0.065mol) in dichloromethane (100ml), add N-methylmorpholine all at once while stirring (8.78g), transfer the clear, colorless solution to the addition funnel. The reactor w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com