Preparation method for vildagliptin impurity
A vildagliptin impurity and compound technology, which is applied in the field of preparation of vildagliptin impurity vildagliptin diketopiperazine, can solve the problems of high cost and complex process, reduce research and development costs, and simplify the synthesis method , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
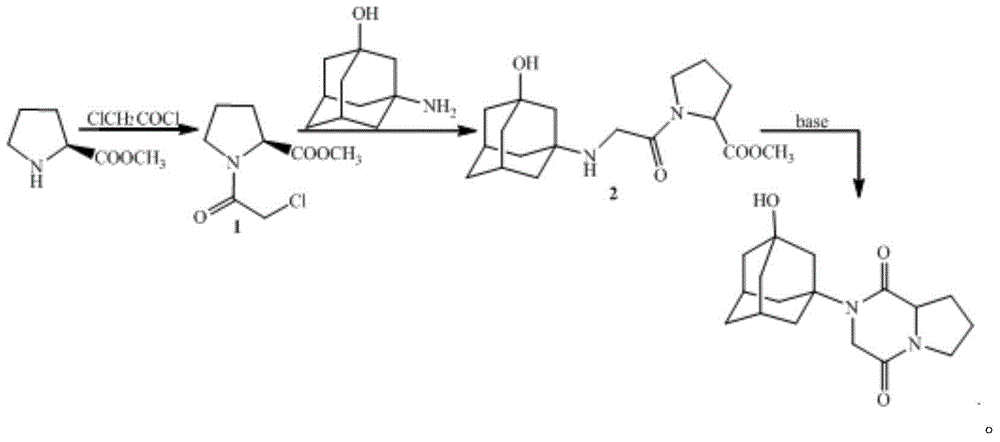
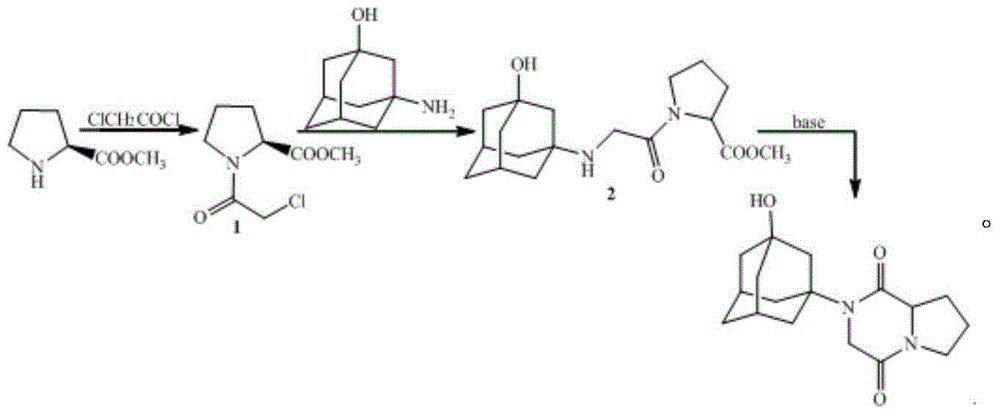
[0034] The preparation of embodiment 1 compound 1
[0035] Add 16.9g (0.15mol) of chloroacetyl chloride to a 1000ml four-necked flask equipped with a mechanical stirrer, a condenser, a thermometer, and a heating device, then add 70ml of dichloromethane, and then add 12.9g (0.1mol) of L-proline Acetate methyl ester and 10.1g (0.1mol) triethylamine were dissolved in 200ml of dichloromethane and slowly added dropwise to the reaction flask, stirred and reacted for 1 hour, added 200ml of water to extract and separate the layers, and then washed the organic phase with 200ml of water, The organic phase was dried and spin-dried to obtain compound 1.
Embodiment 2
[0036] The preparation of embodiment 2 compound 2
[0037] Add 25g (0.15mol) of 3-amino-1-adamantanol, 13.8g (0.1mol) of potassium carbonate, 0.85g of potassium iodide and 200ml of acetone in a 1000ml four-necked flask equipped with mechanical stirring, condenser, thermometer and heating device , heated to reflux, dissolved 20.5g (0.1mol) of compound 1 in 200ml of acetone and slowly added dropwise to the reaction flask, stirred for 1 hour, filtered while hot, spin the filtrate to dryness, added 200ml of water to dissolve, and then added 200ml of di Extract with methyl chloride, wash the organic phase with 200 ml of water, dry the organic phase and spin dry to obtain compound 2.
Embodiment 3
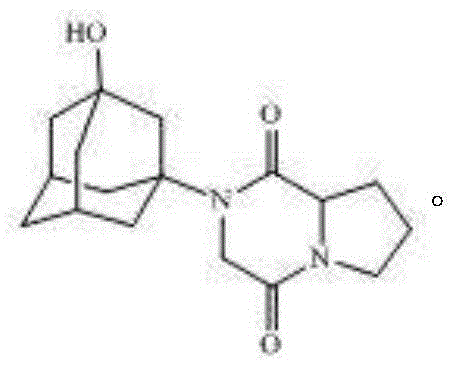
[0038] The preparation of embodiment 3 vildagliptin diketopiperazine
[0039] Add 33.6g (0.1mol) of compound 2 to a 1000ml four-necked flask equipped with a mechanical stirrer, a condenser, a thermometer, and a heating device, then add 13.8g (0.1mol) of potassium carbonate and 200ml of DMF, stir and react at 50°C for 3 hours, filter , adding 300ml of water and 200ml of dichloromethane to extract and separate the layers, wash the organic phase with 200ml of water, separate the layers, dry and spin the organic phase to obtain vildagliptin diketopiperazine with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com