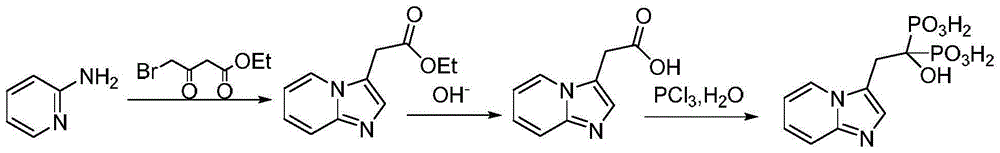
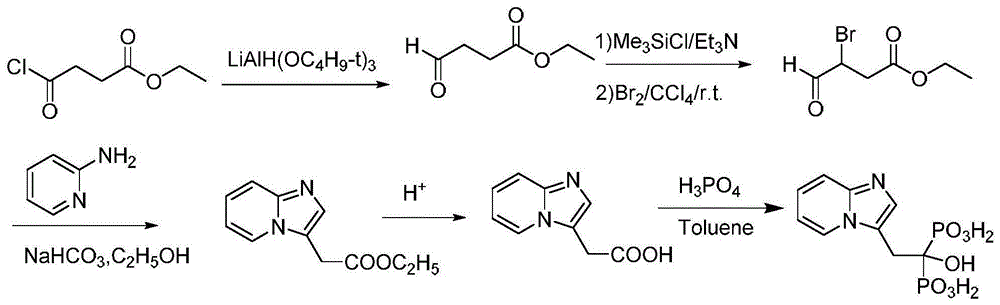
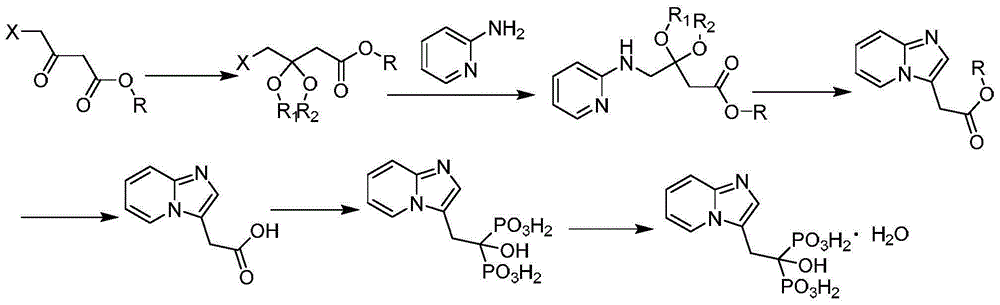
Preparation method of minodronic acid for treating osteoporosis
A technology for minodronic acid and osteoporosis, which is applied in the field of medicine and chemical industry, can solve the problems of slow reaction speed, many by-products, harsh conditions, etc., and achieves the effects of increasing reaction speed, increasing yield and being easy to remove.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0040](1) 14.19g (51.8mmol) of ethyl 3,4-dibromo-butyrate and 1.42g of reaction accelerator A are added in a three-necked flask of a mixed solvent of water and 1,4-dioxane in 100ml, Stir at room temperature for 15 minutes, then add 5.85g (61.16mmol) of 2-aminopyridine and stir at 80°C for 3 hours. TLC monitors that the reaction of ethyl 3,4-dibromo-butyrate is complete, filter, concentrate under reduced pressure, and add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to give 10g of light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl)acetic acid Ethyl ester, the yield is 93.1%, and the purity is 98.5%. Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 4:1, and the mixed solvent is composed of 10ml of water and 30ml of...
Embodiment 2
[0044] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0045] (1) 14.19g (51.8mmol) of 3,4-dibromo-ethyl butyrate and reaction accelerator A1.14g are added in the there-necked flask of the mixed solvent of 100ml water and 1,4-dioxane, Stir at room temperature for 15 minutes, then add 6.34g (67.34mmol) of 2-aminopyridine and stir at 85°C for 5 hours. TLC monitors that the reaction of ethyl 3,4-dibromo-butyrate is complete, filter, concentrate under reduced pressure, and add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to give 10.2g of a light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl) Ethyl acetate, the yield is 94.7%, and the purity is 98.2%. Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 4.5:1, and the mixed solvent is formed by mixing 15ml of water and 30ml of...
Embodiment 3
[0049] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0050] (1) 3,4-dibromo-butyric acid ethyl ester 14.19g (51.8mmol) and reaction accelerator A1.28g are added in the there-necked flask of the mixed solvent of 100ml water and 1,4-dioxane, Stir at room temperature for 15 minutes, then add 7.31g (77.7mmol) of 2-aminopyridine and stir at 70°C for 4 hours. TLC monitors that the reaction of 3,4-dibromo-butyric acid ethyl ester is complete, filter, concentrate under reduced pressure, add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, and removed the solvent under reduced pressure to obtain 10.07g of light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl)acetic acid Ethyl ester, the yield is 93.2%, and the purity is 97.9%. Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 5:1, and the mixed solvent is composed of 10ml of water and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com