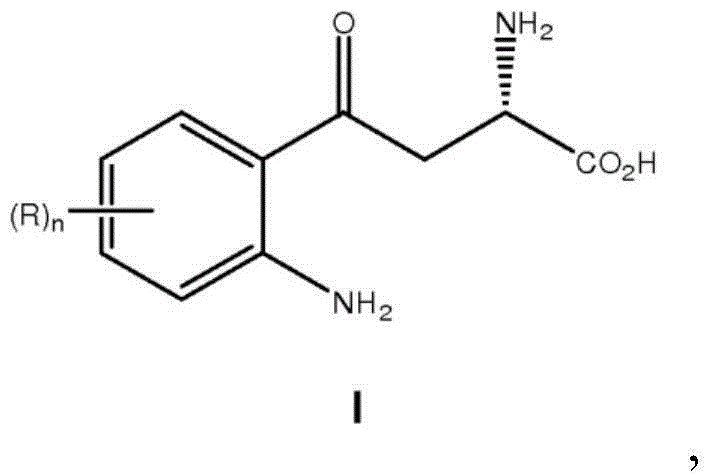
Methods for the synthesis of chiral kynurenine compounds
A technology of compounds and hydrates, applied in the field of synthesizing compounds including chiral kynurenine compounds and related compounds, which can solve the problems of failure, low solubility, failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
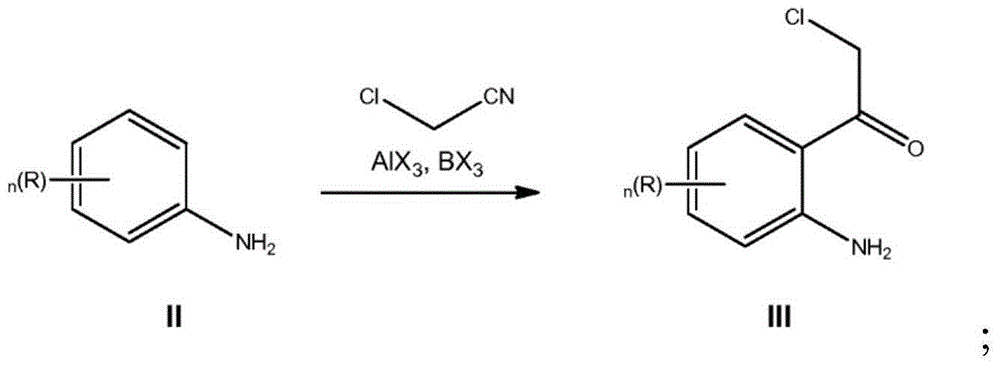
[0095] Preparation of 1-(2-amino-4-chloro-phenyl)-2-chloro-ethanone (IIIa)
[0096] 1MBCl in dichloromethane (45L, 45mol, 1.1 equiv) was dissolved at 0°C jacket temperature 3 Transfer to a reactor and add toluene (13 L). A solution of 3-chloroaniline (IIa, 4.5 L, 42 mol) in toluene (39 L) was added over 38 minutes at -5°C to 3°C, and after 31 minutes, aluminum trichloride (5.8 kg, 43 mol, 1.0 equivalent). After 3 minutes, a solution of chloroacetonitrile (3.4 L, 54 mol, 1.3 equiv) in toluene (3.4 L) was added at -4°C to 6°C during 10 minutes. Heat to 65°C, hold at a maximum jacket temperature of 100°C for 47 minutes, and stir the mixture at 65°C overnight. The reaction mixture was added to 1N aqueous HCl (81 L) at 43°C over 41 minutes and stirred at 48°C for 30 minutes. After cooling to 20° C., the phases were separated and the aqueous phase was extracted twice with dichloromethane (2×40 L). The combined organic phases were washed with water (20.5 L). Dichloromethane (75...
example 2
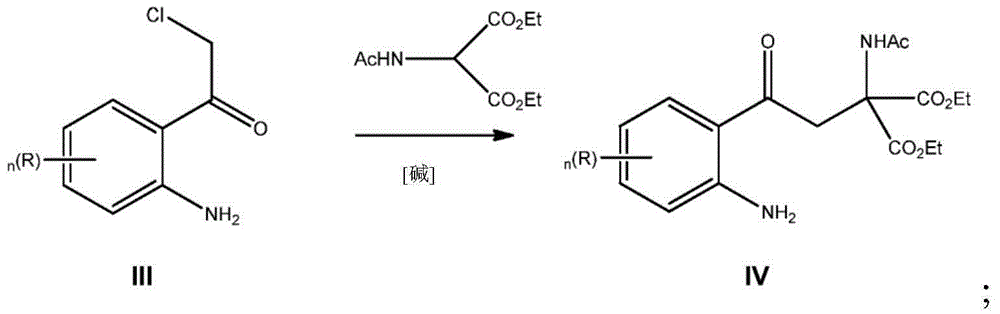
[0098] Preparation of 2-acetylamino-2-[2-(2-amino-4-chloro-phenyl)-2-oxo-ethyl]-diethyl malonate (IVa)
[0099]To a solution of diethyl acetamidomalonate (5.87 kg, 27.0 mol, 1.01 eq) in ethanol (31.5 L) was added 21% sodium ethoxide in ethanol (12.1 L, 32.4 mol, 1.21 eq) at 22°C The solution. 1-(2-amino-4-chloro-phenyl)-2-chloroethanone (IIIa, 5.44kg, 26.7mol), sodium iodide (0.60kg, 4.0mol, 0.15 equivalents), ethanol ( 17L) and tetrahydrofuran (THF, 17L) were dosed into the reactor and stirred at 45°C for 2 hours. After the reaction mixture was evaporated to 45% of the original volume, water (36 L) was added and the aqueous phase was extracted twice with dichloromethane (34.5 L, 20 L). The combined organic phases were washed with water (14 L). After removing 25% of the original volume by distillation under reduced pressure, isopropanol (32 L) was added and 50% of it was distilled off. Heptane (11 L) was added at 60°C. The mixture was cooled to 5 °C, filtered and washed w...
example 3
[0101] Preparation of 2-acetylamino-4-(2-amino-4-chloro-phenyl)-4-oxo-butyric acid (Va)
[0102] Diethyl 2-acetylamino-2-[2-(2-amino-4-chloro-phenyl)-2-oxo-ethyl]-malonate (IVa, 5.80kg, 15.1mol) in water ( A solution in 3.8 L), dioxane (55 L) and 30% NaOH (7.7 L, 76.8 mol, 5.09 equiv) was heated to reflux at a jacket temperature of 110° C. for 45 minutes. Acetic acid (12.0 L) was added at 65-70°C and the suspension was heated to reflux while maintaining a jacket temperature of 110°C for 1.5 hours. Sat aqueous NaCl (30 L) and ethyl acetate (54 L) were added at 20°C, the phases were separated and the aqueous phase was extracted with ethyl acetate / dioxane 1:1 (40 L). The combined organic phases were then washed with saturated aqueous NaCl (14.5 L). The organic phase was evaporated to dryness (product crystallization before evaporation was complete) and stripped with ethanol (15.5 L) to give 2-acetamido-4-(2-amino-4-chloro-phenyl)-4-oxo- Butyric acid (Va, 3.65 kg, 12.8 mol, 85%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com