Application of miRNA in liver cirrhosis diagnosis and treatment
A 1. miRNA-548a-3p, patient technology, applied in the field of biomedicine, can solve problems such as inducing cancer and abnormal gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
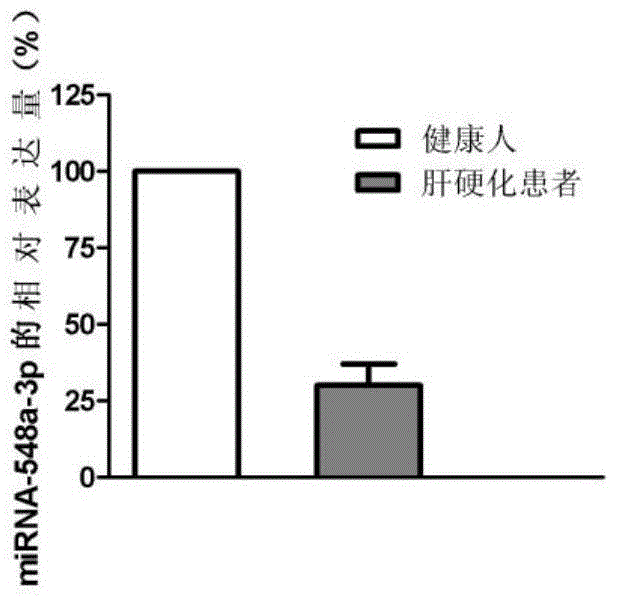
[0044] Example 1 Screening of miRNAs associated with liver cirrhosis
[0045] 1. Sample acquisition: 10 biopsy samples from healthy people and 10 biopsy samples from patients with liver cirrhosis were collected. The acquisition of all the above samples was approved by the ethics committee of the organization.
[0046] 2. Extraction of total RNA from samples
[0047] Total RNA in advance using QIAGEN Tissue RNA Extraction Kit. Specific steps are as follows:
[0048] 1) In a clean area with less RNase interference, use a mortar containing an appropriate amount of liquid nitrogen to weigh about 20 mg of the biopsy tissue sample, and grind it to powder with a pestle;
[0049] 2) Transfer the sample to a 2ml centrifuge tube without RNase;
[0050] 3) Add 300 μl Lysissolution, place in a homogenizer, and grind thoroughly for 1-5 minutes;
[0051] 4) 12000g, 4°C, centrifuge for 10min, transfer the supernatant to a new 1.5ml centrifuge tube;
[0052] 5) Add 600 μl RNase-FreeWate...
Embodiment 2
[0075] Example 2 QPCR verification of differentially expressed miRNA-548a-3p
[0076] 1. Select miRNA-548a-3p according to the detection results of the miRNA chip for large sample QPCR verification. According to the sample collection method in Example 1, 80 cases of biopsy tissue samples from patients with liver cirrhosis and 80 biopsy samples from healthy people were selected.
[0077] 2. The RNA extraction process is the same as in Example 1.
[0078] 3. Reverse transcription:
[0079] 1) Prepare the reaction system according to Table 1
[0080] Table 1 reaction system
[0081] RNA template
1μg
10× buffer
2μl
dATP (10mM)
2μl
polyA polymerase
0.5μl
RNase inhibitor
0.5μl
wxya 2 o
Make up to 20μl
[0082] Incubate at 37°C for 1h.
[0083] 2) Add 1 μl of 0.5 μg / μl Oligo(dT) specific RT primer to the reaction tube, and incubate at 70° C. for 5 minutes.
[0084] 3) Immediately incubate on ice for 2 ...
Embodiment 3
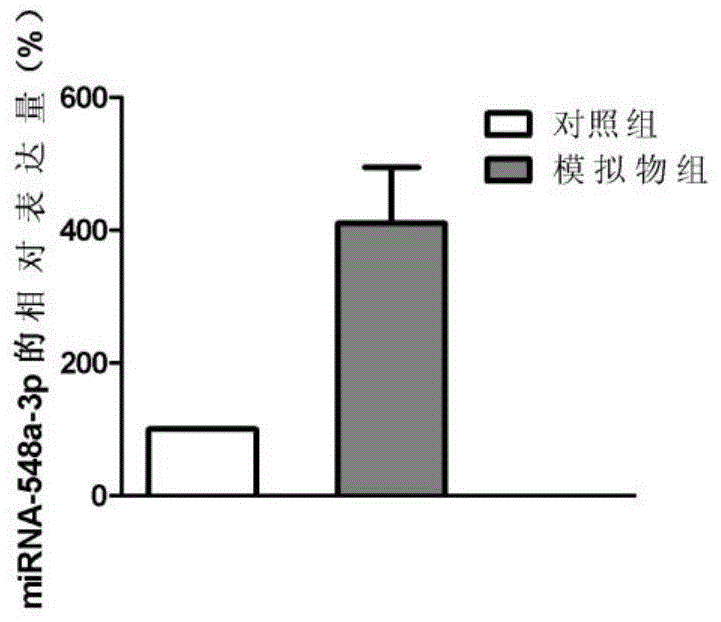
[0101] Example 3 Study on the effect of miRNA-548a-3p on the proliferation ability of liver cirrhosis cells
[0102] 1. Design and synthesis of mimics targeting miRNA-548a-3p
[0103] According to the sequence information of miRNA-548a-3p, miRNA-548a-3p mimics and random control sequences were designed and synthesized by Dalian Bao Biotechnology Co., Ltd.
[0104] 2. Cell culture
[0105] Hepatic stellate cells LX-2 were incubated with DMEM high-glucose medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in an incubator.
[0106] 3. Cell transfection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com