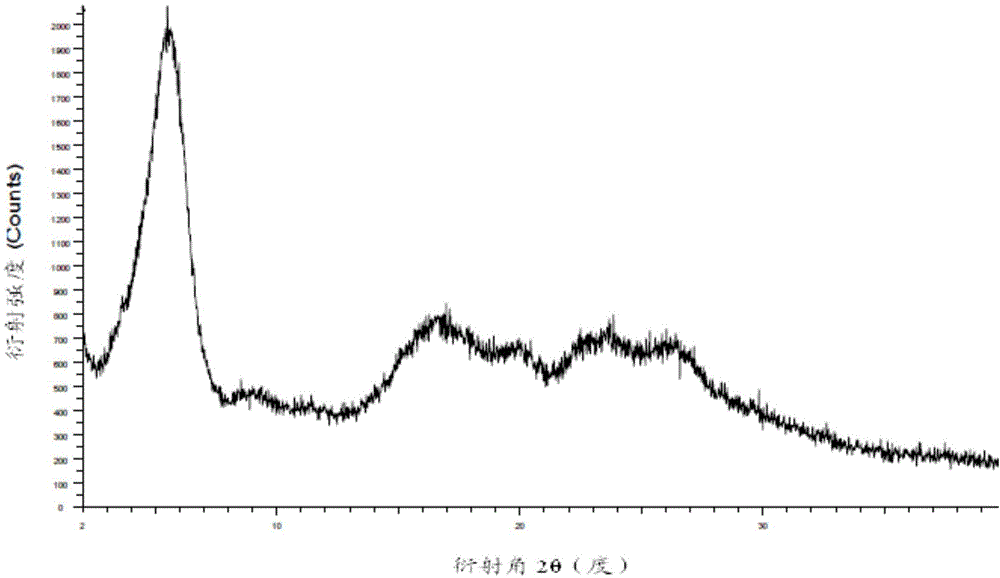
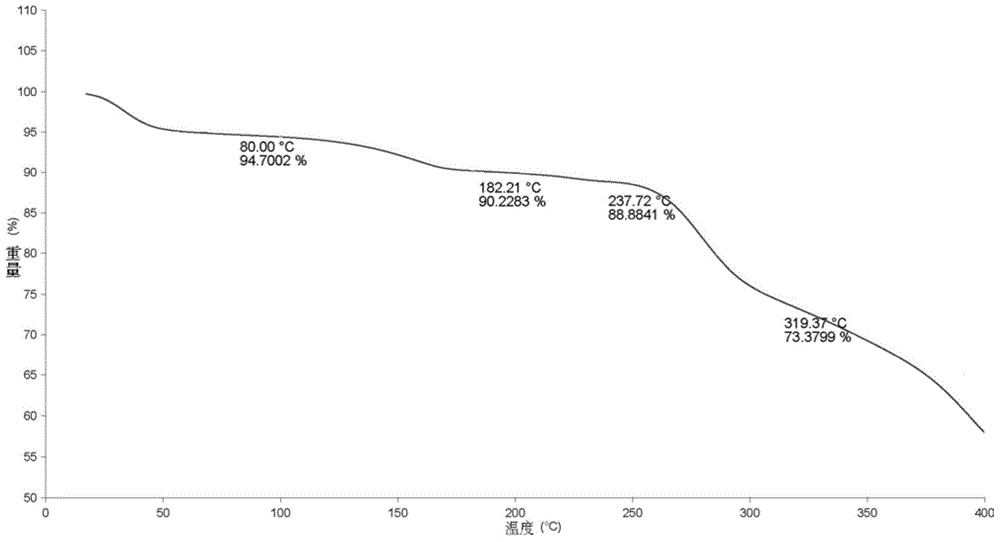
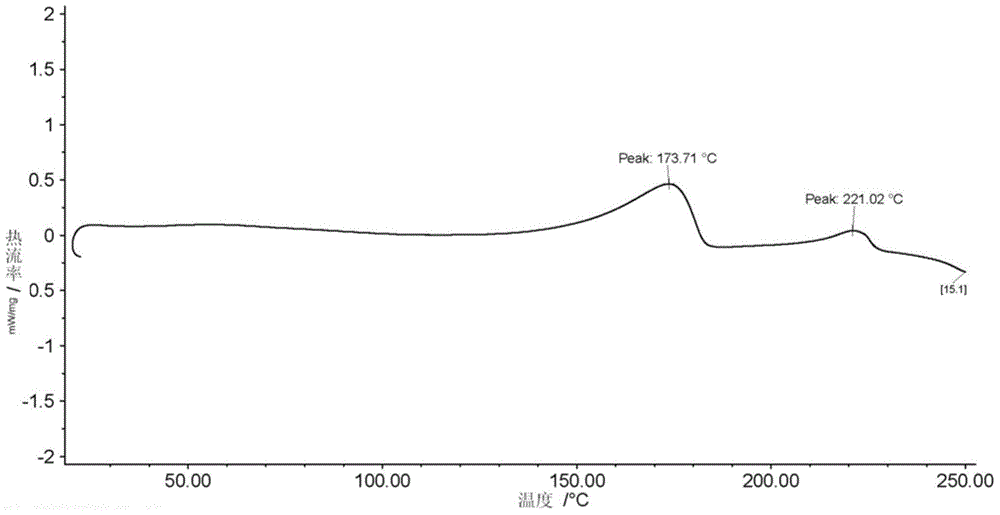
Preparing method for regadenoson crystal form E
A technology of regadeson and crystal form, which is applied in the field of preparation of regadeson crystal form E, and can solve the problem of low purity of crystal form E
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention discloses a method for preparing crystal form E of regadepine, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0037] The abbreviations and English specific meanings used in the specification and claims are as follows:
[0038]
[0039]
[0040] The raw materials or reagents used in the pre...
Embodiment 1
[0047] Embodiment 1 Preparation of Regardson Crystal Form E
[0048]Dissolve 40 mg of regadenoson in 2 mL of DMSO to obtain a 20 mg / mL solution. Filter through a 0.45μm filter membrane, and inject the liquid into the 2mL quantitative loop of the reversed-phase chromatography system through the injection needle. The reversed-phase chromatography conditions are: chromatographic column is C18, 250×4.6mm; UV wavelength is 254nm; It is acetonitrile, phase A is water phase, and the flow rate is 1mL / min; the gradient elution method is adopted, and the gradient elution procedure is as follows:
[0049] time
0-5min
5%
5-10min
5%-10%
10-20min
15%
20-40min
15%-50%
40-45min
50%-90%
[0050] Separation was carried out according to the above established procedure, and the main peak sample with a retention time of 30 min was collected to obtain a mixture of acetonitrile and water. Add 10 mL of dichlor...
Embodiment 2
[0059] Embodiment 2 Preparation of Regardson crystalline form E
[0060] Dissolve 10 mg of regadenoson in 1 mL of DMF to obtain a 27 mg / mL solution. Filter through a 0.45μm filter membrane, and inject the liquid into the 2mL quantitative loop of the reversed-phase chromatography system through the injection needle. The reversed-phase chromatography conditions are: chromatographic column is C18, 250×4.6mm; UV wavelength is 254nm; It is acetonitrile, phase A is water phase, and the flow rate is 1mL / min; the gradient elution method is adopted, and the gradient elution procedure is as follows:
[0061] time
[0062] Separation was carried out according to the above established procedure, and the main peak sample with a retention time of 30 min was collected to obtain a mixture of acetonitrile and water. Add 50 mL of ethyl acetate to the obtained mixture, separate the organic phase, concentrate under reduced pressure at 30°C, add 1 mL of DMF to the residue, dissolve it,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com