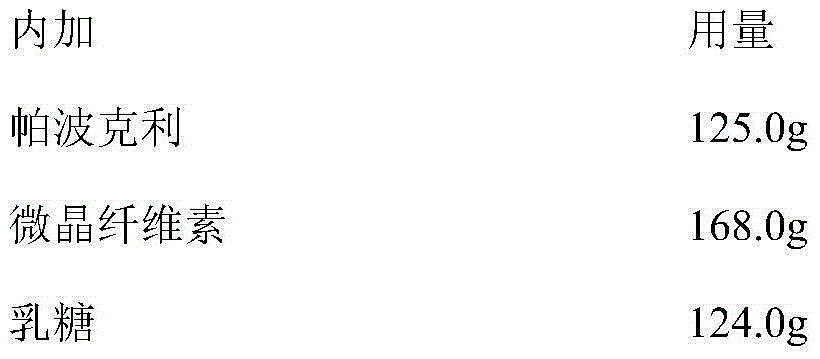Pharmaceutical composition prepared by dry granulation technology
A dry granulation and composition technology, which is used in drug combinations, antitumor drugs, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This example prepares the pharmaceutical composition with Papoclide free base. (1000 formulation units)
[0028]
[0029]
[0030] Add Papoclite free base, colloidal silicon dioxide, microcrystalline cellulose, sodium starch glycolate, magnesium stearate (added internally), and lactose, and mix well. After mixing, the material is put into the dry granulator for granulation. The equipment parameters are: oil pressure 35-50Kg / cm 2 , Feeding 8Hz, tablet pressing 18Hz, granulation 10Hz, granulation aperture 1mm. Take the granules and powder after granulation, add magnesium stearate (additional), mix well, compress into tablets or fill capsules.
Embodiment 2
[0032] This example prepares the pharmaceutical composition with Papoclide free base. (1000 formulation units)
[0033]
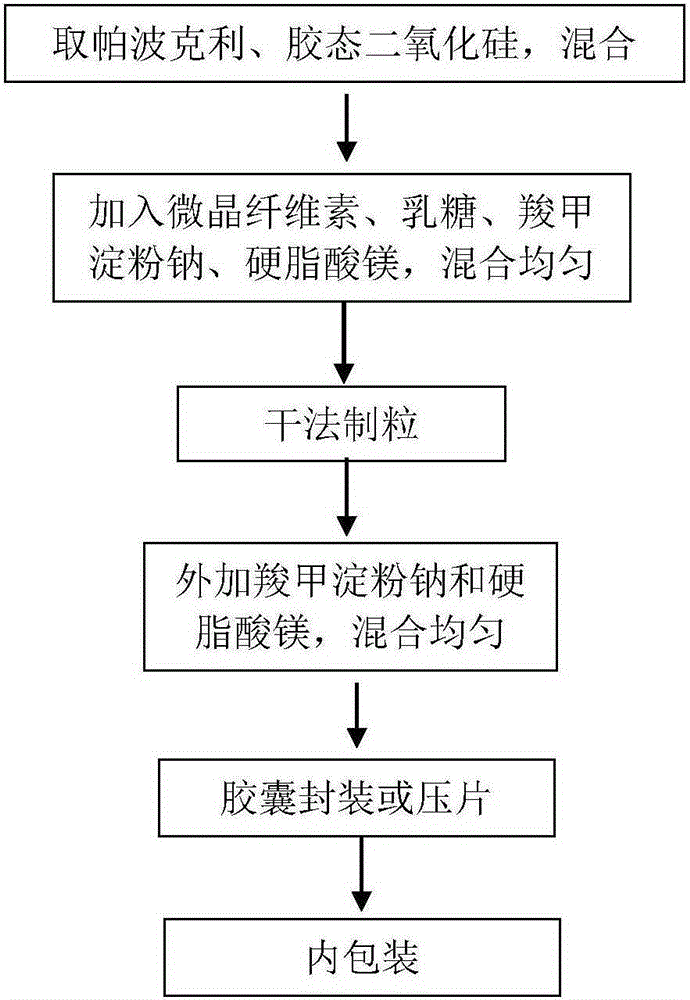
[0034] Combine Papoclite free base, colloidal silicon dioxide, and mix well. Add microcrystalline cellulose, sodium starch glycolate, magnesium stearate (added in), lactose, and mix well. After mixing, the material is put into the dry granulator for granulation. The equipment parameters are: oil pressure 35-50Kg / cm 2 , Feeding 8Hz, tablet pressing 18Hz, granulation 10Hz, granulation aperture 1mm. After granulation, add sodium carboxymethyl starch (additional) and magnesium stearate (additional) to the granules and powder, mix well, press into tablets or fill capsules, see the process flow chart figure 1 .
Embodiment 3
[0036] This example prepares the pharmaceutical composition with Papoclide free base. (1000 formulation units)
[0037]
[0038]
[0039] Combine Papoclite free base, colloidal silicon dioxide, and mix well. Add microcrystalline cellulose, sodium starch glycolate, magnesium stearate (added in), lactose, and mix well. After mixing, the material is put into the dry granulator for granulation. The equipment parameters are: oil pressure 35-50Kg / cm 2 , Feeding 8Hz, tablet pressing 18Hz, granulation 10Hz, granulation aperture 1mm. After granulation, add sodium carboxymethyl starch (additional) and magnesium stearate (additional) to the granules and powder, mix well, press into tablets or fill capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com