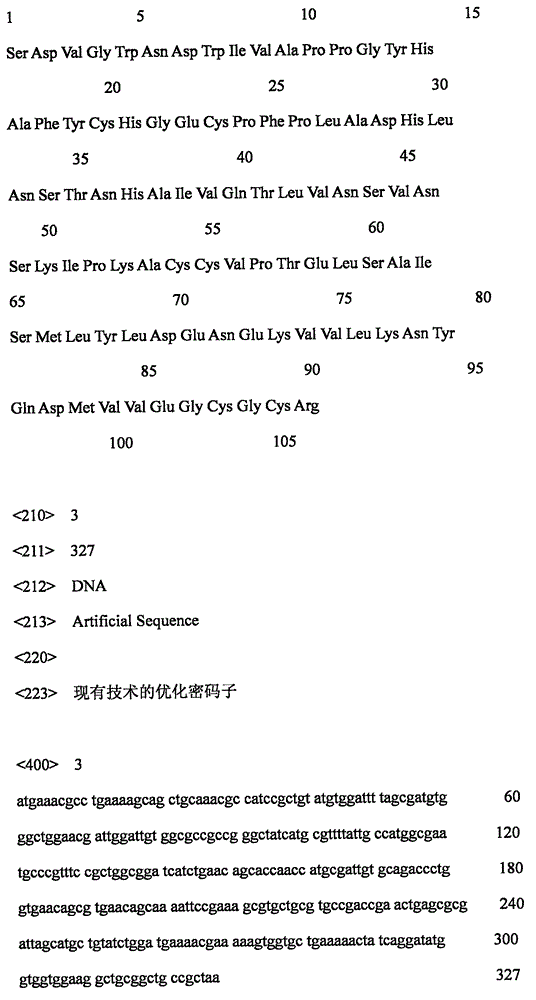Human bone morphogenetic protein 2 mature peptide and expression of mature peptide
A protein and bone cell technology, which is applied to peptide/protein components, animal/human peptides, peptide sources, etc., can solve the problems that no one continues to study BMP2, etc., and achieve the effect of improving curative effect and increasing expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Expression of rhBMP2 of the present invention
[0021] The present inventors discovered rhBMP2 whose amino acid sequence is shown in SEQ ID NO: 2, and carefully designed its expression-optimized coding gene (nucleotide shown in SEQ ID NO: 1) to be expressed and prepared in E. coli. In short, Shanghai Jierui Biotechnology Co., Ltd. was commissioned to artificially synthesize the gene, and then ligated into the pEVT vector (NewEnglandBiolabs) between the EcoRI and HindIII restriction sites, and transformed into E. coli BL21 (DE3). Screened on 50 mg / L ampicillin LB plate, the expression vector contained in the positive bacteria was named pEVT-hBMP2, the plasmid was extracted, and the sequencing was correct.
[0022] The positive bacteria were inoculated into 5mL LB medium, cultured at 30°C and 200r / min for 12h, and then inoculated into 400mL LB medium at 2% (V / V) inoculum, and cultured at 30°C, 200r / min for 8h. Then fermentation is carried out, and the 400mL fermente...
Embodiment 2
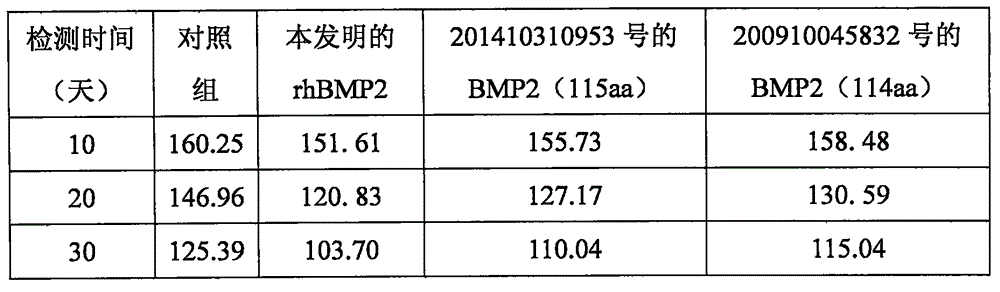
[0025] Example 2 Experiment of promoting bone formation of rhBMP2 of the present invention
[0026] The rhBMP2 of the present invention and the BMP2 of Chinese Patent Application No. 201410310953 and Chinese Patent Application No. 200910045832 were used to conduct a bone differentiation calcification test. Specifically, take the bone marrow fluid of 1 SD rat (male) and add it to DMEM culture medium containing 200ml / L fetal bovine serum, 100U / ml penicillin and 100Lg / ml streptomycin at 37℃, 50ml / L Cell culture was carried out with carbon dioxide, and the culture medium was changed every 3 days until the bone marrow stromal cells were 80% confluent, digested with 2.5g / L trypsin, and subcultured at a ratio of 1:2. Take the bone marrow stromal cells passed down to the 4th generation to 10 4 The amount per well was inoculated into a 96-well plate, the culture medium was discarded after 24 hours, and then randomly divided into 4 groups (all groups on the same 96-well plate, a total of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com