Site 2 insulin analogues
A technology of insulin analogs and insulin, applied in the direction of insulin, hormone peptides, drug combinations, etc., can solve problems such as disturbing modification or substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
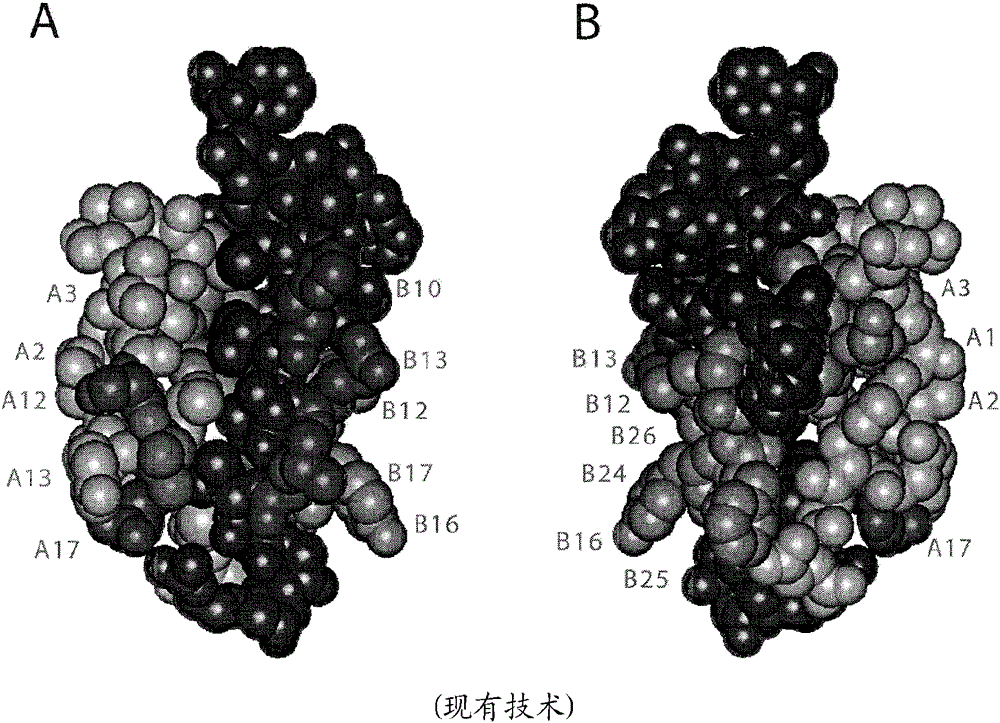
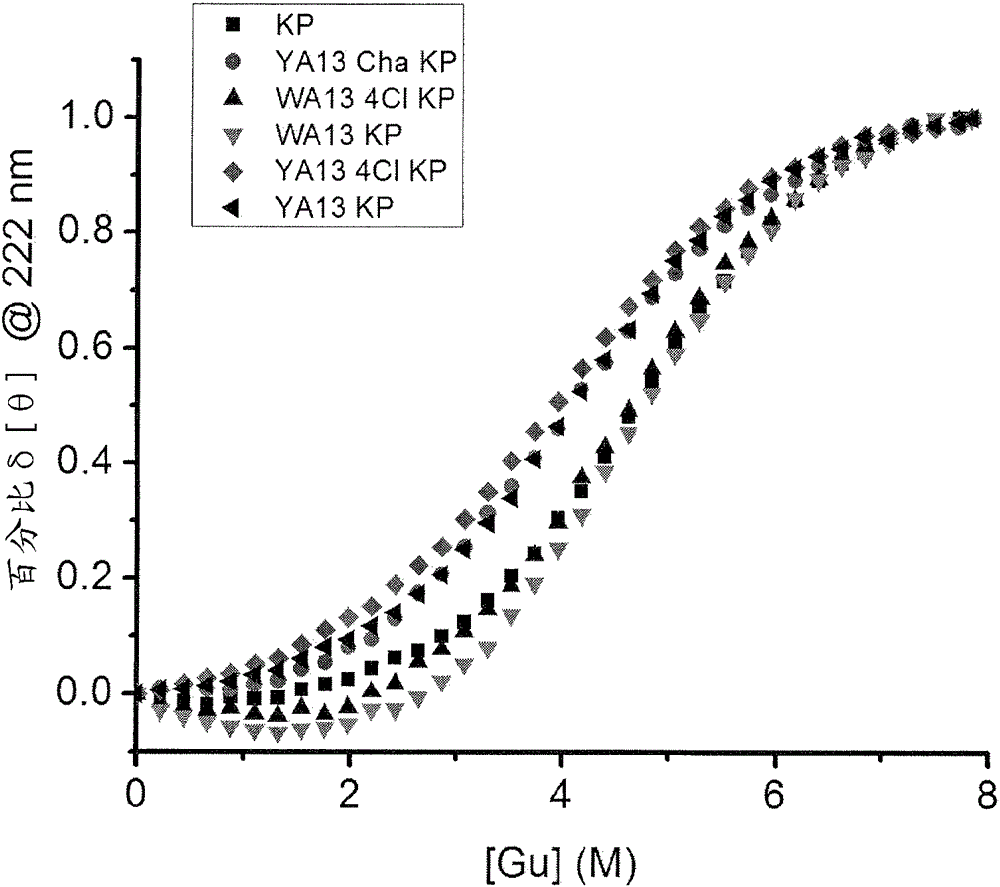
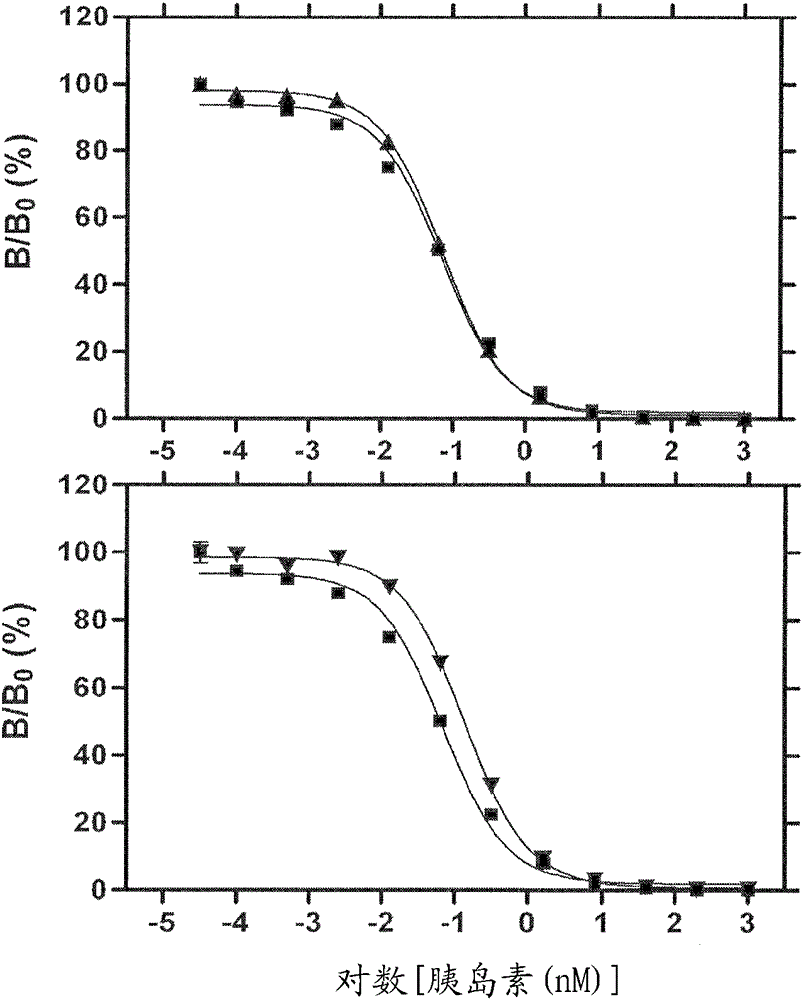
[0026] The present invention relates to double-chain or single-chain insulin analogues which provide (i) rapid absorption from subcutaneous depots, and (ii) shortened duration of action with similar ratios of IR-A / IR-B receptor binding affinities that of wild-type insulin with an absolute affinity in the range of 5-100% (the lower limit was chosen to correspond to proinsulin). Examples of B-chain substitutions that confer rapid absorption are aspartic acid or lysine at position B28, optionally in combination with proline at position B29. Removal of the proline from position B28 was associated with reduced intensity of dimerization and hexamer assembly independent of the nature of the substituted amino acid. Yet another example of a B-chain substitution that confers rapid absorption is the combination of lysine at position B3 and glutamic acid at position B29 when formulated in the absence of zinc ions. Amino acid substitutions introduced to achieve reduced signaling duration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com