Rifamycin S preparing method
A technology of rifamycin and ethanol, which is applied in the direction of organic chemistry, can solve the problems of simple production process, low consumption, and recyclability, and achieve the effects of easy promotion and use, saving production cost and simplifying production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
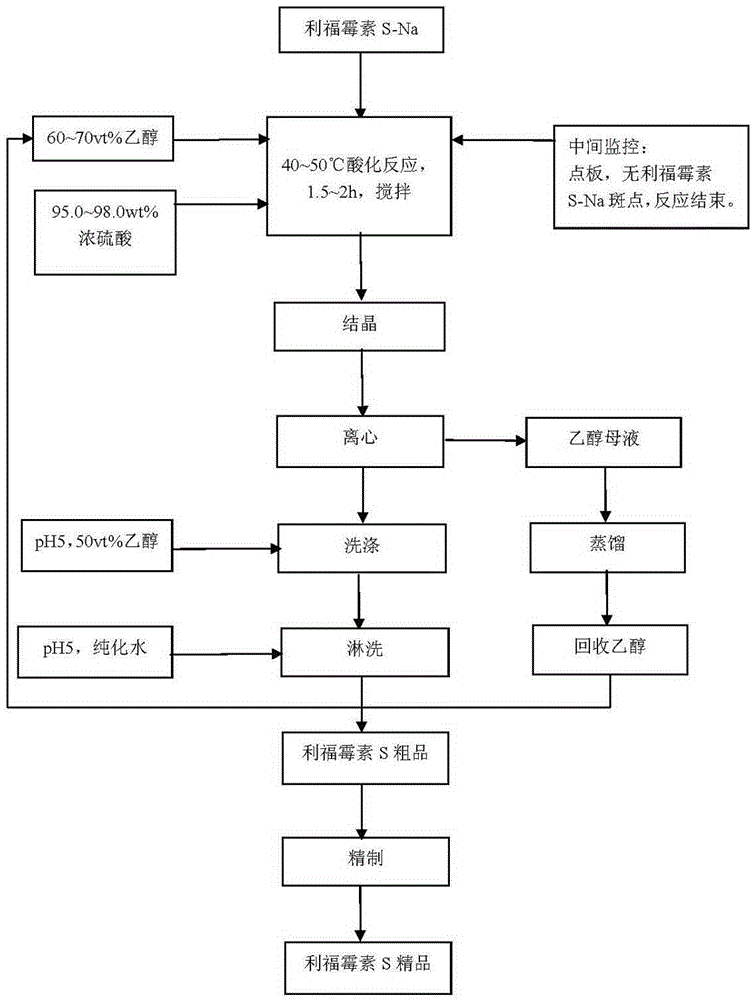
[0024] The preparation method of this rifamycin S comprises the following steps:
[0025] 1) Using rifamycin S-Na as the starting material, 65vt% ethanol and 95.0~98.0wt% concentrated sulfuric acid as the solvent, under acidic conditions, stir at 40~50°C for 1.5~2h, point the plate during the stirring process, until No rifamycin S-Na spots, the reaction is over;
[0026] Each material mass ratio is rifamycin S-Na: ethanol: purified water: sulfuric acid=100:180:120:8.5;
[0027] 2) Reduce the temperature of the mixture obtained in step 1) to 30°C at a cooling rate of 5°C / h, keep the temperature for 20 minutes, and then drop to room temperature, then lower the temperature to 5°C at a cooling rate of 3°C / h, stop stirring, and let it stand for more than 2 hours , and then centrifuged to obtain a solid mixture and a mother liquor, loosen the crude product of rifamycin S, pickle and wash with water to obtain the crude product of rifamycin S;
[0028] Pickling: Use sulfuric acid to...
Embodiment 2
[0036]In step 1), the mass ratio of each material is rifamycin S-Na:ethanol:purified water:sulfuric acid=100:226:120:9.0, the concentration of ethanol is 70vt%, other steps are the same as in Example 1.
Embodiment 3
[0038] In step 1), the mass ratio of each material is rifamycin S-Na:ethanol:purified water:sulfuric acid=100:145:120:8.0, the concentration of ethanol is 60vt%, and other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com