Application of podophyllotoxin isatin derivative to anti-leukemia drug and preparation method for podophyllotoxin isatin derivative
A technology for podophyllotoxin and derivatives, applied in the field of podophyllotoxin isatin derivatives and their synthesis, can solve problems such as multidrug resistance, bone marrow suppression and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
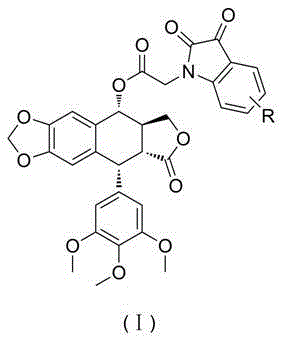
[0034] Preparation of 4-α-[2-chloro-acetate]-podophyllotoxin (b)
[0035] Add podophyllotoxin (a) (2.4mmol), triethylamine (9.6mmol) and dichloromethane (20ml) into a 50ml round-bottomed flask, stir, add chloroacetyl chloride (6mmol) dropwise under ice-cooling, dropwise, The reaction solution was raised to room temperature and stirred for reaction. After the reaction was completed, the reaction was quenched with saturated ammonium chloride solution, extracted three times with dichloromethane, the organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and column chromatography gave light yellow solid 4-α-[2-chloro-acetic acid Esters]-podophyllotoxin (b) 0.95g, yield 81%.
[0036] 1 HNMR (400MHz, CDCl 3 )δ6.77(s,1H),6.55(s,1H),6.37(s,2H),5.99(d, J =4.8Hz,2H),5.96(d, J =8.4Hz,1H),4.61(d, J =2.6Hz,1H),4.38-4.42(m,1H),4.13-4.24(m,3H),3.80(s,3H),3.75(s,6H),2.84-2.97(m,2H); 13 CNMR (100MHz, CDCl 3 )δ 173.41, 167.94, 152.65, 148.41, 147.70, 137....
Embodiment 2
[0038] Preparation of 4-α-[2-(1H-indol-2,3-dione-1-yl)-acetate]-podophyllotoxin (I-1)
[0039] Add chloroacetate podophyllotoxin (0.2mmol), potassium carbonate (0.2mmol), isatin (0.19mmol) and N,N-dimethylformamide (2ml) in a 10ml round bottom flask, and heat the oil bath to The reaction was stirred at 80°C. After completion of the reaction, water (20ml) was added to quench the reaction, extracted three times with dichloromethane, the organic layer was washed with saturated ammonium chloride and saturated brine, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and column chromatography gave an orange-red solid 4 -α-[2-(1H-indol-2,3-dione-1-yl)-acetate]-podophyllotoxin (I-1) 0.087g, yield 73%.
[0040] 1 HNMR (400MHz, CDCl 3 )δ7.63~7.69(m,2H),7.21(t, J =7.0Hz,1H),6.84(d, J =6.6Hz,1H),6.59(s,1H),6.52(s,1H),6.33(s,2H),5.99(d, J =9.6Hz,2H),5.94(d, J =8.1Hz,1H),4.70(d, J =17.2Hz,1H),4.59(s,1H),4.53(d, J =17.1Hz,1H),4.33~4.36(m,1H),4.16(t...
Embodiment 3
[0042] Preparation of 4-α-[2-(4-chloro-1H-indol-2,3-dione-1-yl)-acetate]-podophyllotoxin (I-2)
[0043] Replace isatin with 4-chloroisatin, according to the method described in Example 2, all the other required raw materials and reagents are the same as in Example 2, to obtain orange-red solid 4-α-[2-(4-chloro-1H-indole -2,3-Diketone-1-yl)-acetate]-podophyllotoxin (I-2), yield 70%.
[0044] 1 HNMR (400MHz, CDCl 3 )δ7.55(t, J =8.0Hz,1H),7.15(d, J =8.2Hz,1H),6.75(d, J =7.9Hz,1H),6.55(s,1H),6.52(s,1H),6.32(s,2H),5.99(dd, J =6.1,1.1Hz,2H),5.93(d, J =8.6Hz,1H),4.72(d, J =17.8Hz,1H),4.59(d, J =3.9Hz,1H),4.52(d, J =17.8Hz,1H),4.32~4.36(m,1H),4.16(t, J =9.6Hz,1H),3.80(s,3H),3.72(s,6H),2.86~2.95(m,2H);HRMScalcdforC 32 h 27 ClNO 11 [M+H] + 636.1267, found 636.1271; 13 CNMR (100MHz, CDCl 3 )δ178.85,173.23,167.23,157.19,152.66,150.94,148.49,147.72,138.69,137.10,134.60,134.45,132.53,126.78,126.21,114.88,109.86,107.88,106.49,101.81,70.99,60.77,56.13,45.40,43.57 ,41.54,38.43...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com