Transgenic non-human organisms with non-functional TSPO genes
A non-functional, non-human technology, applied in the direction of organic active ingredients, neurotransmitter receptors, animal/human proteins, etc., can solve the problem of expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0391] Example 1: Development of TSPO knockout animals
[0392] Materials and methods
[0393] Construct design and transgenic animal production
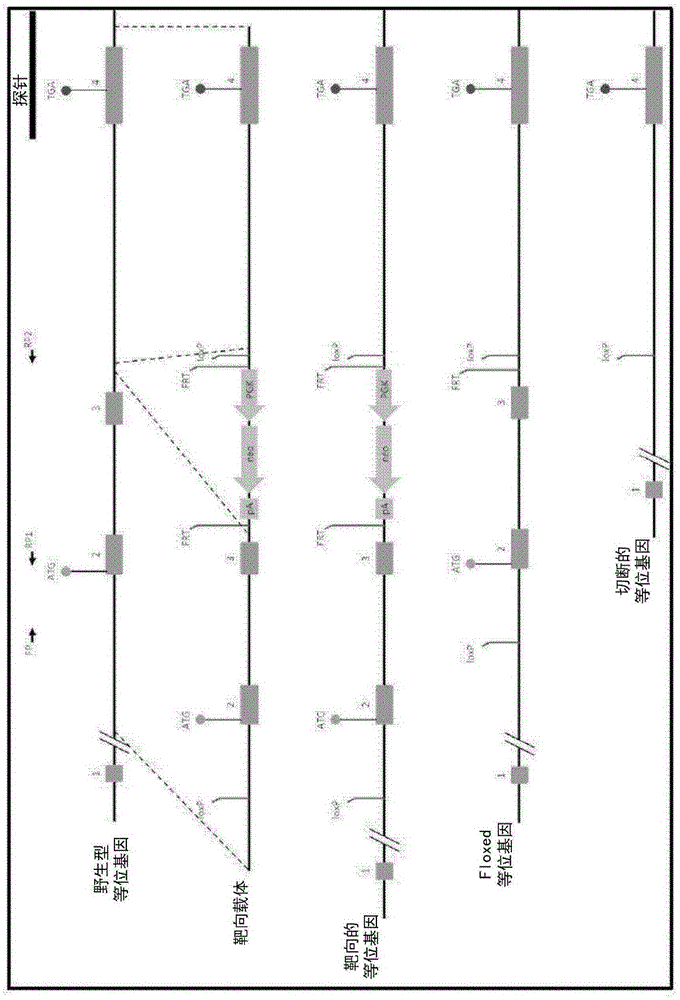
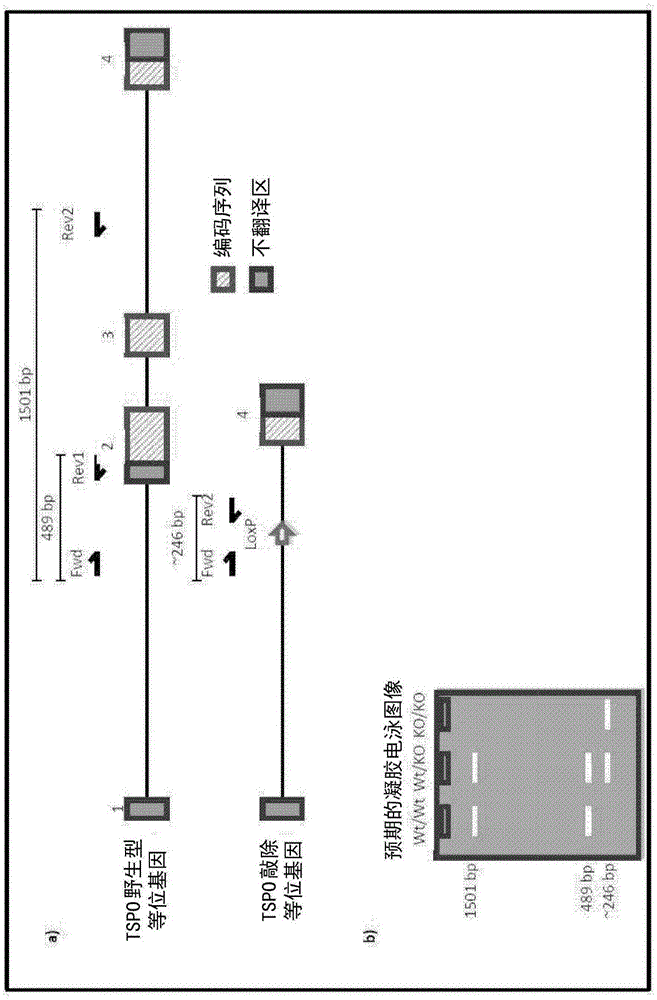
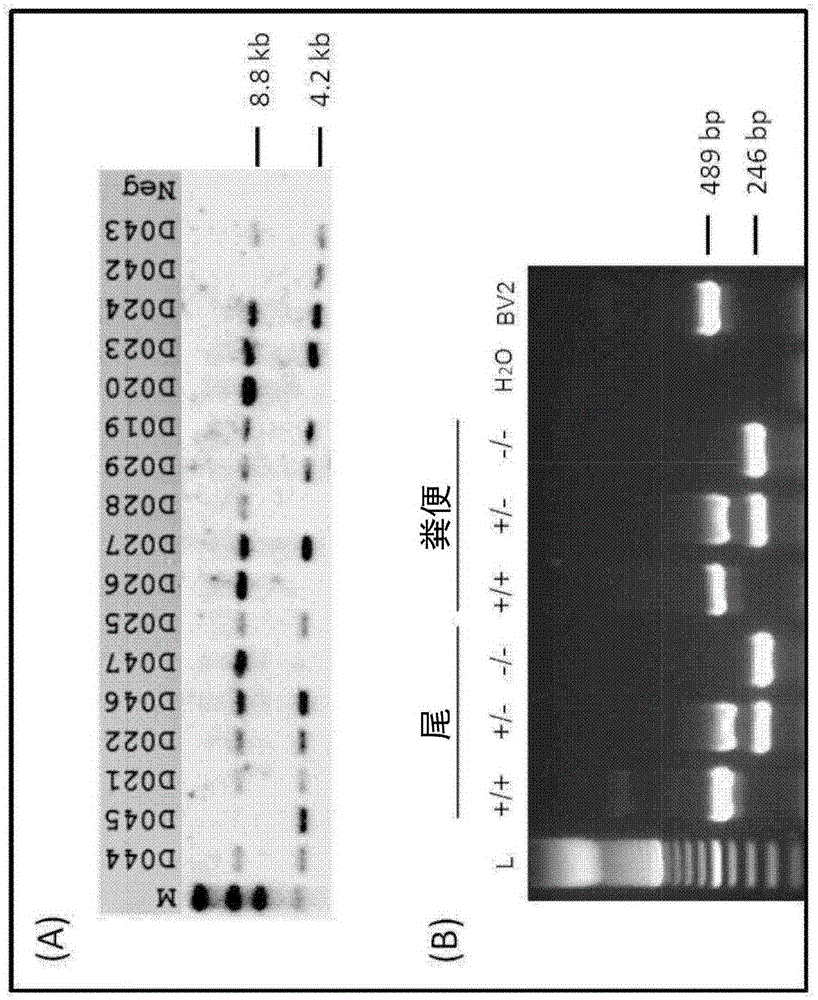
[0394] Development of TSPO knockout mice was performed by Ozgene (Bentley DC, WA, Australia). TSPO knockout mice were generated using the Cre-Lox recombination method. To generate TSPO knockout mice, targeting constructs were generated with some added homology to the wild-type TSPO allele (see figure 1 ). The construct also contained a pair of LoxP sites flanking exons 2 and 3, which contained the TSPO initiation codon, a neomycin cassette to select for the targeting construct, and a flippase recognition target (FRT) successful acquisition of the site. An FRT site was included to allow generation of conditional knockout mice at a later stage. The neomycin cassette confers neomycin resistance to cells that successfully incorporate the construct. The construct was delivered by electroporation into Bruce4 mouse embryonic stem (E...
Embodiment 2
[0419] Example 2: Radioligand Membrane Binding
[0420] Materials and methods
[0421] Membrane preparation
[0422] Tissue samples were homogenized with a T25 digital Ultra-Turrax homogenizer (Ika, Wilmington, NC, USA) in approximately 45 mL of ice-cold TRIS buffer (pH 7.4) at a speed setting of 5 or 20000 rpm, by centrifugation at 48000 g Collect, then discard the supernatant. This step is then immediately repeated as an additional wash step to remove any soluble interfering substances bound by the radioligand (Byland et al. 1993). After a second centrifugation and removal of the supernatant, the samples were resuspended in approximately 50 volumes of ice-cold TRIS buffer (pH 7.4). Aliquots of samples were stored at -80°C until use.
[0423] Protein Concentration Measurement
[0424] Protein concentrations were measured using the bicinchoninic acid (BCA) protein assay kit (ThermoFisher Scientific, Scoresby, VIC, Australia) following the supplier's instructions. Briefly...
Embodiment 3
[0432] Example 3: Analysis of TSPO levels in tissues by autoradiography
[0433] Materials and methods
[0434] Snap-frozen tissues were sectioned at 20 μm in a cryostat, thaw-mounted on poly-L-lysine-coated slides, and stored at −80° C. until the day of the experiment. On the day of the experiment, slides were thawed at room temperature and air-dried with a cold stream. Total and non-specific binding was determined by incubation with 1 nM 3H-PK11195 at room temperature for 20 minutes in the presence or absence of 3 μM of PK11195 in 130 mM TRIS-HCl buffer (pH 7.4). After incubation, slides were briefly submerged twice in 130 mM TRIS-HCl buffer and washed twice in fresh 130 mM TRIS-HCl for 5 minutes at room temperature. Finally, place the slide in cold distilled water (H 2 Rinse briefly 3 times in O), air dry under a cold stream, and allow to dry overnight. Sections were exposed to Kodak BioMaxMR film as well as trace amounts of tritium with known activity concentrations in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com