Preparation method of compound fenbendazole tablet
A fenbendazole tablet and a fenbendazole technology are applied in the field of preparation of recurrent fenbendazole tablets, and can solve problems such as toxic and side effects of the body, inability to achieve effects, increase in drugs, and the like, and achieve good effects, No toxic and side effects, good drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
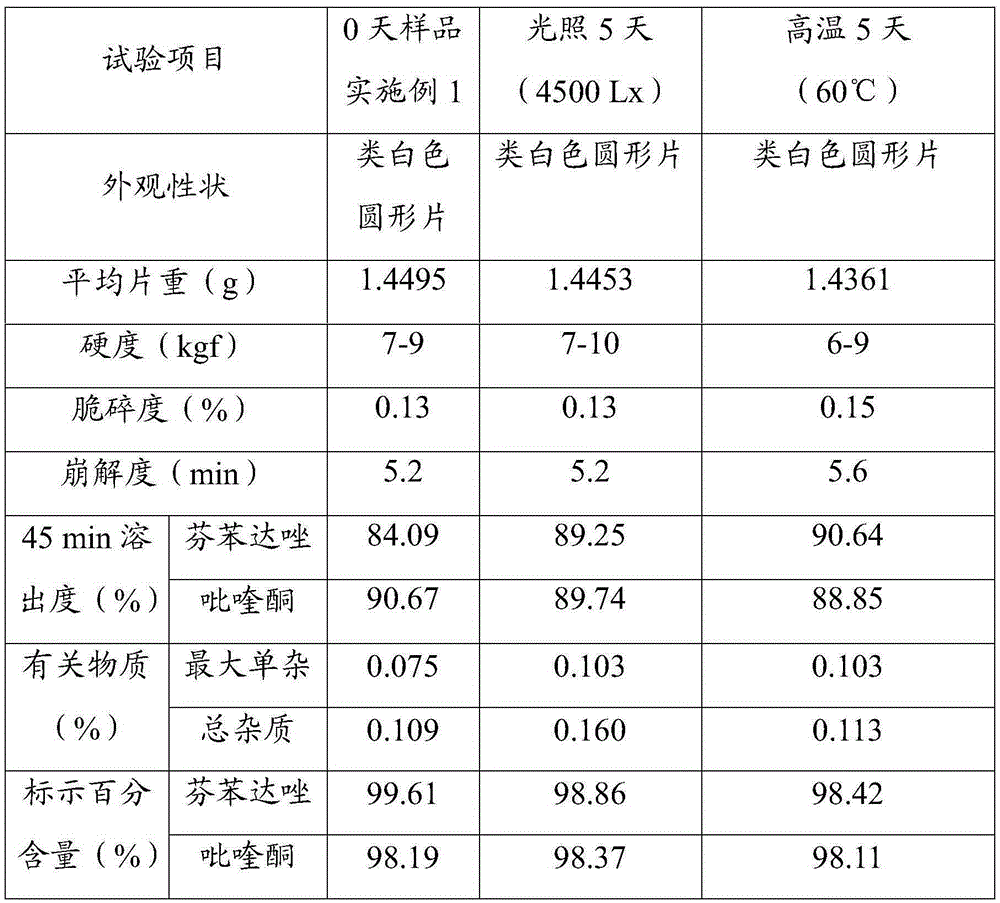
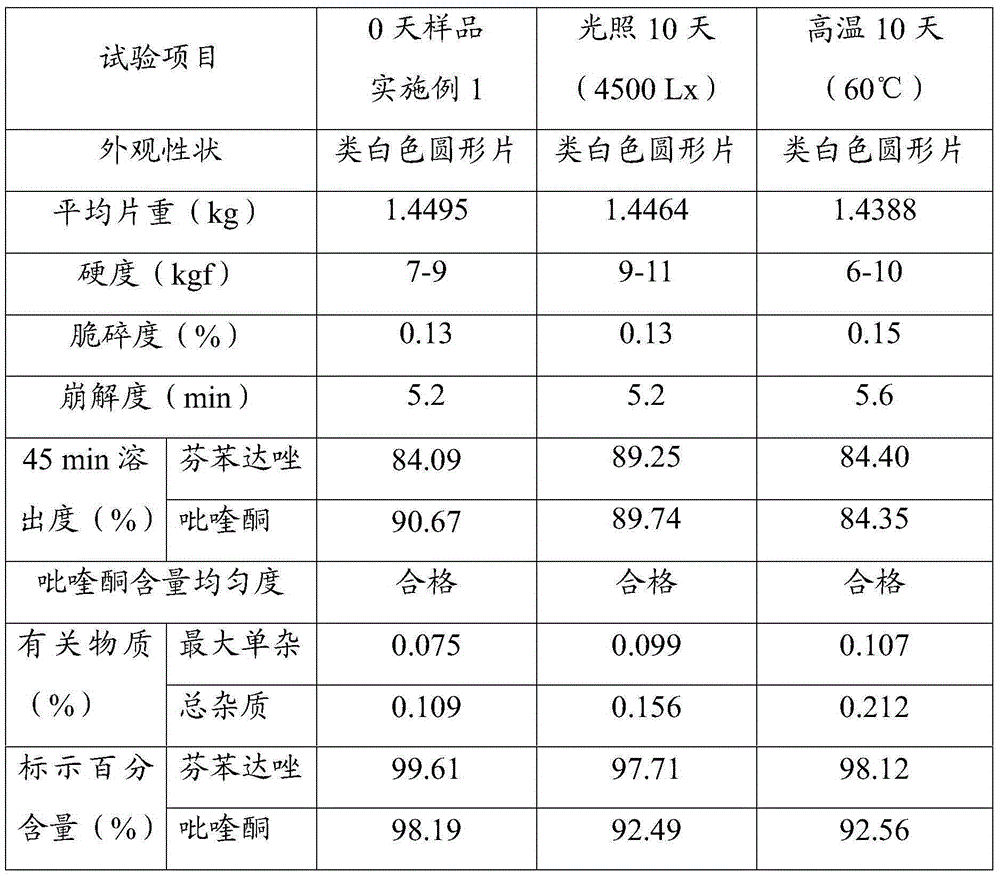
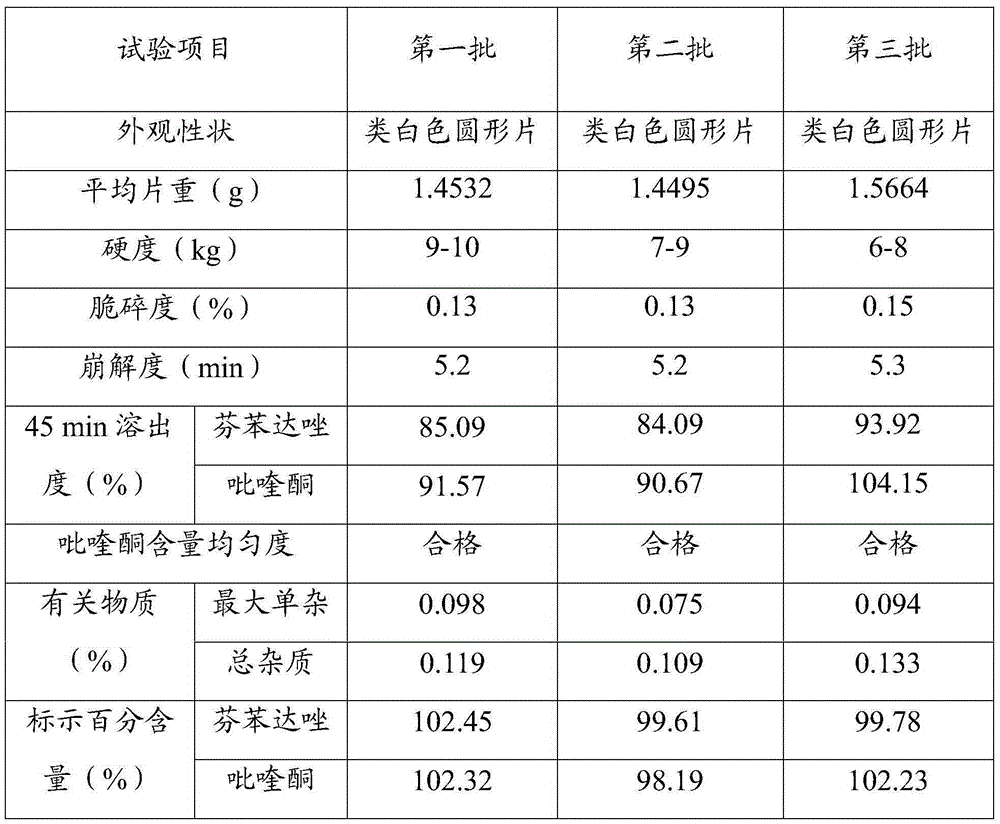
[0023] Three batches of 1000 tablets of compound fenbendazole tablets were prepared according to the determined formula composition and preparation process.
[0024] Each batch of formula consists of: 454g of fenbendazole, 23g of praziquantel, 0.027g of ivermectin, 908g of starch, 90mg of L-HPC, 50mg of arabic mucilage and 100mg of gelatin pulp, 15g of 1% micronized silica gel and Spartan 1.5g.
[0025] making process:
[0026] The first step, after mixing fenbendazole and starch, superfinely pulverize, pass through a 200-mesh sieve, and set aside the mixture, and pulverize praziquantel and ivermectin respectively, pass through a 100-mesh sieve, set aside;
[0027] In the second step, add 40ml of anhydrous gelatin to the ivermectin crushed in the first step, and ultrasonically dissolve it completely, and set aside;
[0028] The third step, the praziquantel is mixed with the mixture of the first step in a three-dimensional mixer according to the equal amount of incremental me...
Embodiment 2
[0048] Prepare 1000 compound fenbendazole tablets according to the determined formula composition and preparation process.
[0049]Each batch of formula composition: 300mg of fenbendazole, 15mg of praziquantel, 0.01mg of ivermectin, 600mg of starch, 80mg of L-HPC, a mixed solution of 25mg of Arabic mucilage and 75mg of gelatin pulp, 10mg of 1% micronized silica gel and aspirin Patan 1mg.
[0050] making process:
[0051] The first step, after mixing fenbendazole and starch, superfinely pulverize, pass through a 150-mesh sieve, and set aside the mixture, praziquantel and ivermectin are ground and pass through a 100-mesh sieve, respectively, and set aside;
[0052] In the second step, add 20ml of anhydrous gelatin to the ivermectin crushed in the first step, and ultrasonically dissolve it completely, and the obtained ivermectin gelatin solution is ready for use;
[0053] In the third step, mix praziquantel with the mixture obtained in the first step in a three-dimensional mixe...
Embodiment 3
[0059] Prepare 1000 compound fenbendazole tablets according to the determined formula composition and preparation process.
[0060] The composition of each batch of formula: 500mg of fenbendazole, 30mg of praziquantel, 0.05mg of ivermectin, 1000mg of starch, 120mg of L-HPC, a mixed solution of 100mg of arabic mucilage and 100mg of gelatin pulp, 20mg of 1% micronized silica gel and aspirin Patan 3mg.
[0061] making process:
[0062] The first step, after mixing fenbendazole and starch, superfinely pulverize, pass through a 200-mesh sieve, and set aside the mixture, pulverize praziquantel and ivermectin and pass through a 120-mesh sieve, respectively, and set aside;
[0063] In the second step, add 40ml of anhydrous gelatin to the ivermectin crushed in the first step, and ultrasonically dissolve it completely, and the obtained ivermectin gelatin solution is ready for use;
[0064] The third step is to mix praziquantel with the mixture obtained in the first step in a three-dim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com