Phospholipid compound of hydrophilic drugs as well as pharmaceutical composition and application of phospholipid compound
A technology of phospholipid compounds and hydrophilic drugs, applied in the field of medicine, can solve problems such as poor lipophilicity and poor transmembrane ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Synthesis of Gemcitabine Derivatives Protected by tert-Butoxycarbonyl (t-BOC)
[0132] Synthesize 4-N-BOC-3'-O-BOC-gemcitabine (4- N-3'-O-Bis(tert-Butoxycarbonyl) gemcitabine, 1), 3'-O-BOC-5'-O-BOC-gemcitabine (3', 5'-O-Bis(tert-Butoxycarbonyl) gemcitabine, 2), 4-N-BOC-5'-O-Boc-gemcitabine (4-N-5'-O-Bis(tert-Butoxycarbonyl) gemcitabine, 3).
Embodiment 2
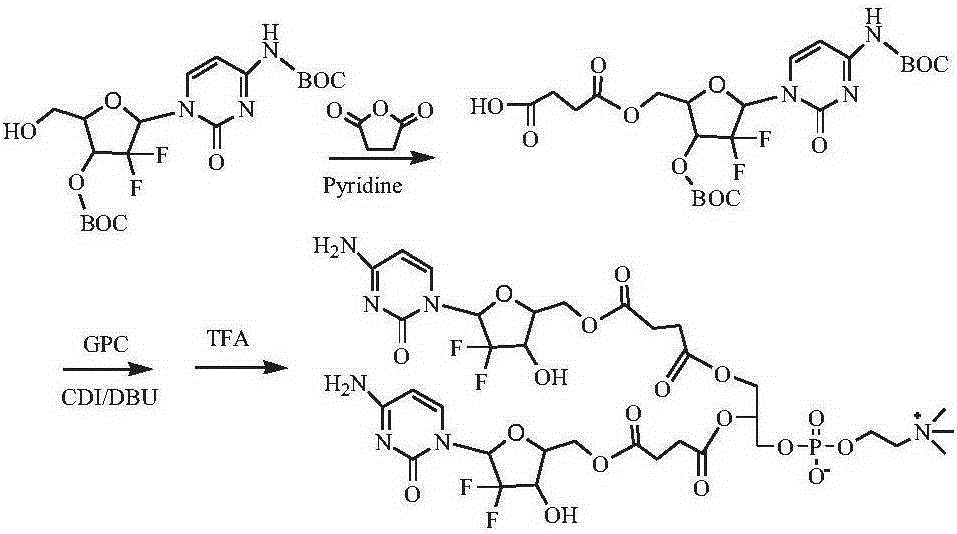
[0134] Synthesis of two (gemcitabine-5'-succinate) phosphatidylcholine compounds (see the synthetic route figure 1 )
[0135] Dissolve 1g of 4-N-BOC-3′-O-BOC-gemcitabine in dichloromethane, add 1.5g of pyridine, 2g of succinic anhydride, react at 40°C for 8 hours, filter, remove the solvent by rotary evaporation, and obtain 4- N-BOC-3'-O-BOC-gemcitabine-5'-succinic acid monoester 0.85 g. Dissolve 0.8g of 4-N-BOC-3′-O-BOC-gemcitabine-5′-succinic acid monoester in dimethyl sulfoxide, add CDI0.5g, activate for 1h, add GPC0.4g and DBU0.5g, room temperature After 24 hours of reaction, a precipitate was precipitated in cold ether, and separated by column chromatography to obtain 0.66 g of bis(4-N-BOC-3'-O-BOC-gemcitabine-5'-succinate)phosphatidylcholine. Bis(4-N-BOC-3′-O-BOC-gemcitabine-5′-succinate) phosphatidylcholine was dispersed in chloroform, TFA was added dropwise at 0°C, raised to room temperature and reacted for 3 hours, desorption After removing the BOC protecting group...
Embodiment 3
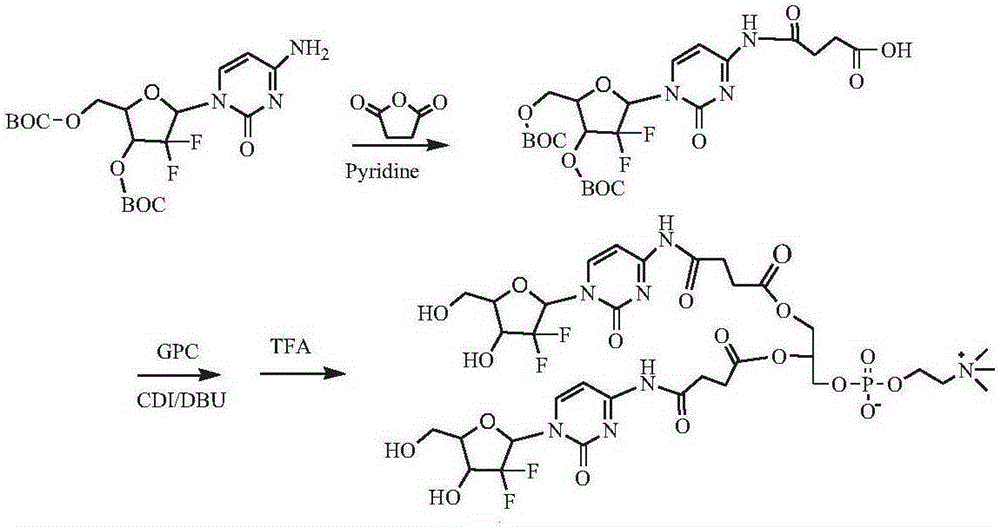
[0139] Synthesis of two (gemcitabine-4-N-succinyl) phosphatidylcholine compounds (see the synthetic route figure 2 )
[0140] Dissolve 1 g of 3′-O-BOC-5′-O-BOC-gemcitabine in dichloromethane, add 1.5 g of pyridine and 2 g of succinic anhydride, react at 0°C for 8 hours, filter, remove the solvent by rotary evaporation, and separate by column chromatography to obtain 3 '-O-BOC-5'-O-BOC-gemcitabine-4-N-succinic acid 0.81 g. Dissolve 0.8g of 3′-O-BOC-5′-O-BOC-gemcitabine-4-N-succinic acid in dimethylsulfoxide, add 0.5g of CDI, activate for 1h, add 0.4g of GPC and 0.5g of DBU, and keep at room temperature After 24 hours of reaction, a precipitate was precipitated in cold ether, and separated by column chromatography to obtain 0.56 g of bis(3'-O-BOC-5'-O-BOC-gemcitabine-4-N-succinyl)phosphatidylcholine. Bis(3′-O-BOC-5′-O-BOC-gemcitabine-4-N-succinyl)phosphatidylcholine was dispersed in chloroform, and TFA was added dropwise at 0°C, raised to room temperature and reacted for 3 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com